Mitochondrial-arginine Theory of Ageing
Research and Production Center «KORVET», Domodedovo, Moscow region, Russia
Author and article information
Cite this as
Kasumov E, Kasumov R, Kasumova I. Mitochondrial-arginine Theory of Ageing. Arch Biochem. 2024; 7(1): 010-013. Available from: 10.17352/ab.000010
Copyright License
© 2024 Kasumov E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Despite the efforts of scientists, the problems of premature ageing, as well as diseases such as cancer, diabetes, herpes zoster, osteoporosis, and others remain unresolved. Guanidine compounds have a strong effect on these processes. We have proposed the mechano-chemiosmotic model, where the electron transfer in the ETC, a cyclic low-amplitude swelling shrinkage of mitochondria, and ATP synthesis are coupled. According to the mechano-chemiosmotic mechanism, energy transformation both in the synthesis of ATP and in the hydrolysis of ATP in the muscles occurs with the direct participation of amino acid residues of arginine and lysine. In addition, arginine and lysine are involved in many processes in the cell metabolism. We believe that ageing begins with a decrease in arginine synthesis due to mitochondrial dysfunction associated with low mobility. It is necessary to maintain the arginine content in the organism by taking it exogenously, and the lysine content of an essential amino acid must be constantly replenished.
Healthy longevity has been the most desired goal of mankind since ancient times and people have been looking for means to achieve this goal. Various theories have been developed to understand the mechanisms of aging, but there is still no clear understanding of not only the mechanisms of aging but also the methods of rejuvenation. Various models for ageing, each focusing on different biochemical and/or cellular pathways have been proposed [1].
Harman’s mitochondrial theory of ageing [2], which is an “extended version” of the free radical hypothesis, is based on the assumption that ageing occurs due to the cumulative effect of free radicals on mitochondrial DNA and its function. According to this theory, mitochondria are the main source of destructive free radicals that attack various components of the cell, and the leakage of free radicals from the respiratory chains of mitochondria occurs almost uncontrollably and constantly. This means that animals with a high metabolic rate generate free radicals quickly and have a short lifespan, while animals with a low metabolic rate do the opposite. Even though this point of view was not justified, the mitochondrial theory of ageing takes into account the exceptional importance of the role of mitochondria in the vital activity of living organisms and ageing and therefore is of great interest. In our opinion, this theory is unable to fully explain the mechanism of ageing due to insufficient knowledge of the mechanisms of mitochondrial functioning. It is known that the Reactive Oxygen Species (ROS) is also formed in young organisms and plays an important physiological role in the cellular processes and development of the organism. For example, a low level of ROS is involved in the selective removal of mitochondria in mitophagy, while a high level is involved in nonselective macroautophagy [3]. It is believed that the reason for the formation of an increased level of ROS is the delay of an electron for more than the optimal time required in the Electron Transport Chain (ETC) in sites up to cytochrome c1 of the inner mitochondrial membrane, where the formed ROS causes chain reactions of lipid peroxidation, damage to mitochondrial DNA, mitochondrial dysfunction, apoptosis, and cell death. However, the reason for the electron delay in the ETC remains unclear. According to the mechano-chemiosmotic model proposed by us, the electron transfer in the ETC, a cyclic low-amplitude swelling shrinkage of mitochondria, and ATP synthesis are coupled [4,5].
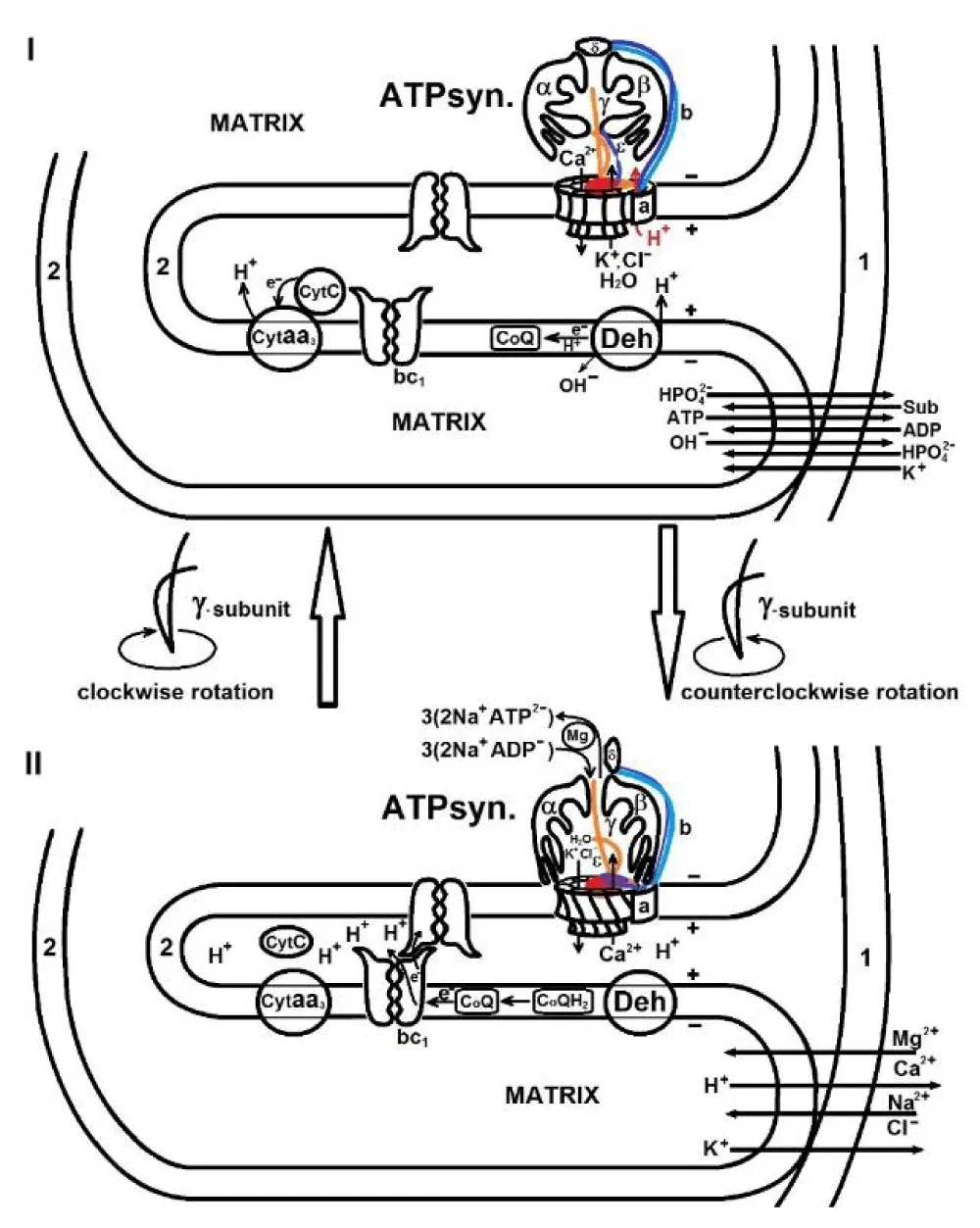
According to this model, as shown in Figure 1 and animation [5], when the intracristal space of mitochondria shrinks, an electron is transferred from the [2Fe-2S] cluster of one dimer to the heme c1 of another dimer of the cytochrome bc1 complex located on the opposite side of the cristae membrane, and when the intracristal space swells, electron transfer stops, and this performs the regulatory role.
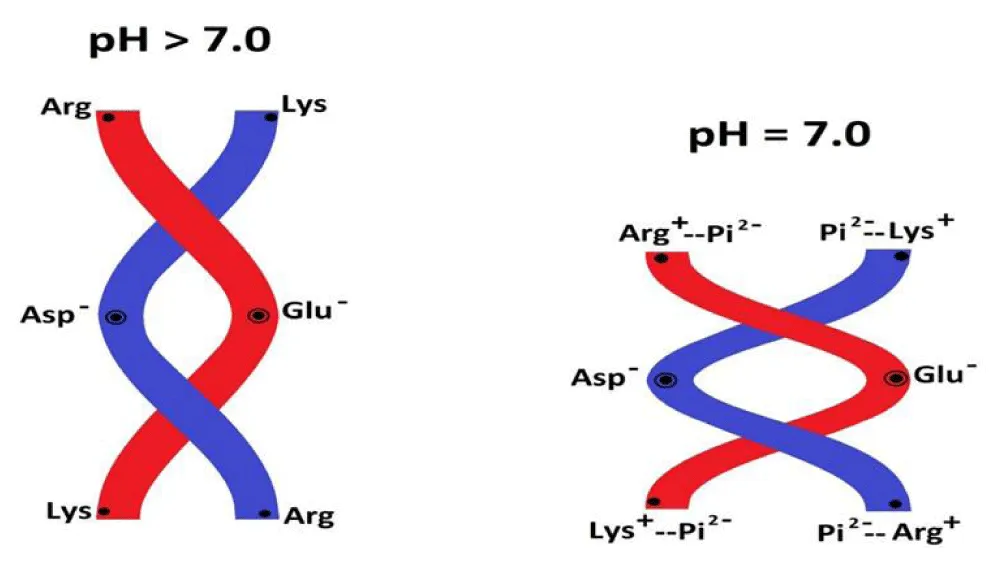
Hyperosmotic conditions, including those caused by water deficiency in the cytosol of old organisms, increase the time of cyclic swelling-shrinkage of mitochondria, which causes a delay in electron transfer in the ETC, a decrease in the rate of ATP synthesis and the formation of ROS. In turn, an increased amount of ROS causes strong depolarization (mild depolarization-repolarization is an integral part of the functioning of mitochondria), the opening of the mPTP, and apoptosis [6]. According to the mechano-chemiosmotic mechanism, cyclic low-amplitude swelling-shrinkage of mitochondria is accompanied by rotation of the γ-subunit and twisting-unwinding of the b2 subunits of ATP synthase, where arginine and lysine residues play a key role. Lysine and arginine residues are involved in energy transformation, both in the synthesis of ATP in mitochondria and in the hydrolysis of ATP in muscles. In Figure 2 (also see an animation [5]) the schematic representation of the contraction and elongation of two arginine and lysine-rich coiled-coil alpha-helical strands (or actin filaments) in the dependence on the pH is demonstrated. L-arginine is a polar basic amino acid with pKa above 10 in the R chair assuming a protonated (cationic) condition at neutral pH. The binding of phosphate ions to protonated arginines and lysines results in the repulsion of negatively charged actin filaments and their contraction. Since human growth stops after 25- 28 years and growth hormone is stimulated by L-arginine, we believe that the decrease in growth hormone synthesis is associated with a decrease in L-arginine synthesis in the body, and then the deficiency of arginine and lysine leads not only to a lack of energy in the body but also other important functions associated with these amino acids are disrupted. L-arginine plays a role in numerous physiological processes including nitrogen detoxification, immune competence, growth hormone (GH) secretion, insulin secretion, vascular dysfunction with ageing, and cardiovascular disease [7]. Telomeric repeat-binding.
Factor 2 (TRF2) functions to protect telomeres and contains an N-terminal basic domain rich in arginines [8]. Arginine methylation regulates telomere length and stability. The repeated addition of the Nitric Oxide (NO) donor S-nitroso-penicillamine (L-arginine is a substrate for NO synthase, which catalyzes nitric oxide synthesis [9]) significantly reduced endothelial cells senescence and delayed age-dependent inhibition of telomerase activity [8]. High glucose concentrations cause swelling of mitochondria [10], which reduces the effect of hypoxia in cancer cells [11] and causes glycation of protein lysine and arginine residues during ageing [12].
Thus, we propose a mitochondrial-arginine theory of ageing based on a decrease in arginine synthesis and mitochondrial dysfunction with age. Mitochondrial dysfunction, accompanied by a decrease in the frequency of a low-amplitude swelling-shrinkage cycle, is caused by cascade processes occurring genetically determined by a decrease in the amount of water in the body, associated, incl. with an increase in glucose concentration with age; by a deficiency of arginine and lysine; by a decrease in physical activity, leading to an increase in the level of sugars and a decrease in the level of ADP (ADP in the cell is a trigger for the functioning of mitochondria). In turn, due to mitochondrial dysfunction, protein glycation occurs with the increasing concentration of sugars and ROS. So, to achieve healthy longevity, it is necessary: regular physical activity, intake of sufficient water, a balanced diet, taking into account the elimination of arginine and lysine deficiency, sleep, and the absence of distress [13,14].
Proposed examination and test of the Mechano-chemiosmotic model [4].
We are aware that the view presented here is not accepted by another model, but is presented here for further examination and testing, so that we may conclude this important problem. To prove the mechano-chemiosmotic model of coupling, it is necessary to perform further kinetic experiments (it is desirable to simultaneously obtain different parameters) on individual organelles in a millisecond time scale:
- Cyclic volume changes (shrinkage – swelling) of intracristal space of mitochondria;
- Cyclic volume changes (shrinkage – swelling) of the matrix of mitochondria;
- pH changes of intracristal space of mitochondria;
- pH changes of matrix of mitochondria (mitochondrial matrix pH of individual mitochondria undergo spontaneous alkalization transients [15];
- pH changes of the outer medium of mitochondria and cytosol in msec time scale;
- Dependence of the oxidation-reduction of cyt c1 on the swelling-shrinkage of the mitochondrial intracristal space (for example, this dependence may be investigated on mitoplasts);
- Inter-monomer electron transferring from the [2Fe-2S] cluster to hеme c1 between the opposite dimers during shrinkage;
- Polarization-depolarization of mitochondria membrane potentials;
- Movement of potassium and calcium ions between the matrix and the intermembrane space of mitochondria;
- Movement of potassium, calcium, and other ions through FO of ATP synthase in different directions;
- Conformational changes of ATP synthase subunits during shrinkage-swelling of organelles;
- Direction of the rotation of gamma subunit of ATP synthase in energization and de-energization of mitochondria;
- Rotation of c-ring by protons in the presence of pH gradient on different sides of the membrane without ATP and other phosphate compounds.
We believe that in the presence of oxidative substrate, phosphate ions, ADP, and other essential ions for the formation of ATP, in the energization of mitochondria, we will observe the following processes in the sequence:
Polarization of inner membrane→ movement of potassium ions to the matrix, and calcium ions to the intermembrane space→ swelling of the mitochondrial matrix→ shrinkage of intracristal space → reduction of cyt c1→ depolarization of inner membrane→ movement of calcium ions into the matrix, and potassium ions to the intermembrane space → contraction of the mitochondrial matrix → ATP synthesis → swelling of intracristal space→ oxidation of cyt c1→ release of protons into the external medium (cytosol) → a repeat of cycle.
Disruption of these processes will lead to mitochondrial dysfunction and, consequently, to energy deficiency in the body. It should be noted that at present experimental data are proving some points of our mechano-chemiosmotic model.
It was shown that the mitochondrial matrix pH of individual mitochondria undergoes spontaneous alkalization transients [15] and neighboring crista junctions dynamically oppose and separate from each other in a reversible and balanced manner in human cells [16]. Electron transport activity is necessary for the morphological contraction of mitochondria and this transient morphological contraction of the mitochondrial matrix is accompanied by a reversible loss or decrease of inner membrane potential (depolarization) [17].
We wait for further new experiments before concluding the mechanism of ATP synthesis, since mitochondria, the main energy hub of the cell, are highly dynamic organelles, that play essential roles in cell physiology. Mitochondrial function impinges on several signaling pathways modulating cellular metabolism, survival, and health span. Maintenance of mitochondrial function and energy homeostasis requires both the generation of new healthy mitochondria and the elimination of the dysfunctional ones [18].
The role of L-arginine in the body is very significant and multifaceted, as evidenced by numerous studies. The kidney is the main site of endogenous Arg synthesis and is also responsible for the overall metabolism of this amino acid, participating in synthesis, degradation, and reabsorption [19], however, endogenous arginine synthesis does not suffice to compensate for dietary arginine insufficiency [20].
The mechanisms of action of L-arginine as supplements in the processes of aging and aggravated stress states, in which mechanisms of individual physiological reactivity play an important role. This approach can be used as an element of individual therapy or prevention of premature aging processes depending on the different levels of initial reactivity of the functional systems [21].
It should be noted that at present the mitochondrial-arginine theory of aging combines the principles of the different theories, such as mitochondrial, free radical, telomeric, glycation, etc. through the mechano-chemiosmotic mechanism and the functions of arginine in the body. Mechanisms such as telomere depletion, stem cell depletion, macrophage dysfunction, and cellular senescence gradually manifest in the body, significantly increasing the incidence of diseases in elderly individuals. These mechanisms interact with each other, profoundly impacting the quality of life of older adults [22]. Aging is associated with the development of a low-level, systemic, chronic inflammation known as “inflammaging” [23].
Conclusion
We have considered mitochondrial dysfunction, arginine, and lysine deficiency as the basis of energy deficiency during ageing, however, many other factors affect the body during ageing and aging-related diseases, such as diabetes, cancer, inflammation, etc. Chronic inflammatory states can contribute to diseases of aging such as sarcopenia and frailty. It should be borne in mind that disruption of the synchronous functioning of mitochondrial and nuclear DNA (once two different symbiotic organisms) can have a strong impact on ageing in the body. So, to achieve healthy longevity, it is necessary: regular physical activity, intake of sufficient water, a balanced diet, taking into account the elimination of arginine and lysine deficiency, sleep, and the absence of distress.
- Keizer HG, Brands R, Oosting RS, Seinen W. A comprehensive model for the biochemistry of aging, senescence, and longevity. Biogerontology. 2024;25(4):615-626. Available from: https://doi.org/10.1007/s10522-024-10097-8
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298-300. Available from: https://doi.org/10.1093/geronj/11.3.298
- Sinenko SA, Starkova TY, Kuzmin AA, Tomilin AN. Physiological signaling functions of reactive oxygen species in stem cells: From flies to man. Front Cell Dev Biol. 2021;9:714370. Available from: https://doi.org/10.3389/fcell.2021.714370
- Kasumov EA, Kasumov RE, Kasumova IV. A mechano-chemiosmotic model for the coupling of electron and proton transfer to ATP synthesis in energy-transforming membranes: a personal perspective. Photosynth Res. 2015;123:1-22. Available from: https://doi.org/10.1007/s11120-014-0043-3
- Animation. Available from: https://www.youtube.com/watch?v=48jScej4dl0
- Kasumov EA, Kasumov RE, Kasumova IV. The new vision of mitochondrial permeability transition pore opening. World J Adv Res Rev. 2023;18(2):825-828. Available from: https://doi.org/10.30574/wjarr.2023.18.2.0913
- Heffernan KS, Fahs CA, Ranadive SM, Patvardhan EA. L-arginine as a nutritional prophylaxis against vascular endothelial dysfunction with aging. J Cardiovasc Pharmacol Ther. 2010;15(1):17-23. Available from: https://doi.org/10.1177/1074248409354599
- Mitchell TR, Glenfield K, Jeyanthan K, Zhu XD. Arginine methylation regulates telomere length and stability. Mol Cell Biol. 2009;29(18):4918-4934. Available from: https://doi.org/10.1128/mcb.00009-09
- Caneba CA, Yang L, Baddour J, Curtis R, Win J, Hartig S, et al. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014;5(6):e1302. Available from: https://doi.org/10.1038/cddis.2014.264
- Alcántar-Fernández J, González-Maciel A, Reynoso-Robles R, Pérez Andrade ME, Hernández-Vázquez AdJ, Velázquez-Arellano A, et al. High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS One. 2019;14(12):e0226652. Available from: https://doi.org/10.1371/journal.pone.0226652
- Öğünç Keçeci Y, İncesu Z. Mitochondrial oxidative phosphorylation became functional under aglycemic hypoxia conditions in A549 cells. Mol Biol Rep. 2022;49(9):8219-8228. Available from: https://doi.org/10.1007/s11033-022-07400-6
- Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985). 2007;103(6):2068-2076. Available from: https://doi.org/10.1152/japplphysiol.00670.2007
- Kasumov EA, Kasumov RE, Kasumova IV. On the mechano-chemiosmotic mechanism of action of guanidines on functional activity of mitochondria and aging. Organic Chem Curr Res. 2015;4:136. Available from: https://www.longdom.org/open-access/on-the-mechanochemiosmotic-mechanism-of-action-of-guanidinies-on-functional-activity-of-mitochondria-and-aging-28905.html
- Kasumov EA, Kasumov RE, Kasumova IV. Mild depolarization of the inner mitochondrial membrane is a crucial component of the mechanochemiosmotic mechanism of coupling. J Nov Physiother Phys Rehabil. 2020;7(1):033-035. Available from: https://doi.org/10.17352/2455-5487.000075
- Poburko D, Santo-Domingo J, Demaurex N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem. 2011;286(14):11672-11684. Available from: https://doi.org/10.1074/jbc.m110.159962
- Kondadi AK, Anand R, Hänsch S, Urbach J, Zobel T, Wolf DM, et al. Cristae undergo continuous cycles of membrane remodeling in a MICOS-dependent manner. EMBO Rep. 2020;21(1):e49776. Available from: https://doi.org/10.15252/embr.201949776
- Lee H, Yoon Y. Transient contraction of mitochondria induces depolarization through the inner membrane dynamin OPA1 protein. J Biol Chem. 2014;289(17):11862-11872. Available from: https://doi.org/10.1074/jbc.m113.533299
- Markaki M, Tavernarakis N. Mitochondrial turnover and homeostasis in ageing and neurodegeneration. FEBS Lett. 2020 Aug;594(15):2370-2379. Available from: https://doi.org/10.1002/1873-3468.13802
- Pedrazini MC, Martinez EF, dos Santos VAB, Groppo FC. L-arginine: its role in human physiology, in some diseases and mainly in viral multiplication as a narrative literature review. Futur J Pharm Sci. 2024;10:99. Available from: https://fjps.springeropen.com/articles/10.1186/s43094-024-00673-7
- Martí i Líndez AA, Reith W. Arginine-dependent immune responses. Cell Mol Life Sci. 2021;78(13):5303-5324. Available from: https://doi.org/10.1007/s00018-021-03828-4
- Kurhaluk N. Supplementation with L-arginine and nitrates vs age and individual physiological reactivity. Nutr Rev. 2024 Sep 1;82(9):1239-1259. Available from: https://doi.org/10.1093/nutrit/nuad131
- Cai Y, Han Z, Cheng H, Li H, Wang K, Chen J, et al. The impact of ageing mechanisms on musculoskeletal system diseases in the elderly. Front Immunol. 2024 May 7;15:1405621. Available from: https://doi.org/10.3389/fimmu.2024.1405621
- Sendama W. The effect of ageing on the resolution of inflammation. Ageing Res Rev. 2020;57:101000. Available from: https://doi.org/10.1016/j.arr.2019.101000
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
