International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Preparation of nanocellulose and its potential application
1Pulp and Paper Laboratory, Forest Products Division, Forest Research Institute Malaysia (FRIM), 52109 Kepong Selangor, Malaysia
2Renewable Materials and Nanotechnology Research Group, Department of Chemical Engineering, KU Leuven, Campus Kortrijk, Etienne Sabbelaan 53,8500 Kortrijk, Belgium
Author and article information
Cite this as
Jasmania L, Thielemans W (2018) Preparation of nanocellulose and its potential application. Int J Nanomater Nanotechnol Nanomed. 2018; 4(2): 014-021. Available from: 10.17352/2455-3492.000026
Copyright License
© 2018 Jasmania L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Nanostructured cellulose has received tremendous attention due to its inherent unique properties such as high strength, high surface area, flexible surface chemistry, abundant and renewable. The early polymers industry was nearly completely bio-based and cellulose was one of the most important materials. It has only been the Second World War that changed things and propelled petroleum, as the main source material. Due to global awareness on the use of more green products, cellulose offers an alternative to petroleum-based products. This paper presents the general overview of nanocellulose starting from its cellulose structure and properties to preparation of nanocellulose, properties and its potential application.
One of the major revenue contributors of Malaysia economy comes from the timber industry. In 2008, timber and timber products contributed approximately RM22.5 billion of the total exports valued at RM674 billion. In line with the third Industrial Master Plan (IMP3), more emphasis should be given to higher value-added downstream activities. Therefore, it is important to explore more efforts on the development of high-value added products that could generate more revenue to the industry and inherently the country. Nanocellulose is therefore seen as one of the new potential candidates for creating high value added products from lignocellulosic biomass that could be derived from the timber industry.
Cellulose, the most abundant and renewable organic raw material estimated in excess of 75 billion tonnes production worldwide has been used historically to serve mankind for various domestic and industrial applications [1-3]. For the past 150 years, cellulose and its derivatives have found various applications in the field of coatings, optical films, pulp and paper, pharmaceuticals, food and cosmetic additives [4]. As a matter of fact, cellulose continues to fascinate researchers for its potential applications in a diversity of fields due to its availability, sustainability, low cost as well as its unique inherent properties including biodegradability, biocompatibility and high strength. In addition, environmental concerns have also been a driving factor for many research recent endeavours in order to expand the usage scope of cellulose and also to exploit its interesting properties into a multitude of basic and advanced products.
Cellulose
The term ‘cellulose’ was first used in the work by Payen in 1838. As a matter of fact, through his work, the cellulose molecular formula of C6H10O5 was determined. The isolation of cellulose was carried out by reaction of plant fibres with acid and ammonia followed by extraction with water, alcohol and ether [5,6]. However, it was only about 80 years later that the polymeric structure of cellulose was elucidated and proposed by Staudinger [7]. It was revealed that cellulose not only existed as assemblies of D-glucose but interestingly, also that the glucose units were covalently linked forming long molecular chains. This unique polymer has since attracted a lot of attention from researcher and a number of published reviews have been generated [8-17]. The drive towards a more sustainable economy has also led to renewed attention on cellulose for the development of materials and products.
Structure and properties of cellulose
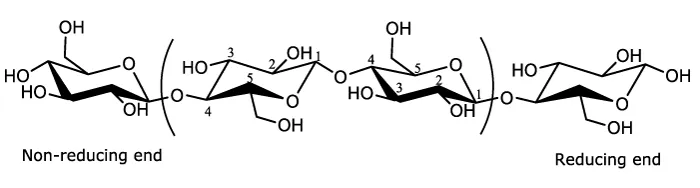
Cellulose is a linear polydisperse homopolysaccharide with a ribbon-like conformation. Cellulose is built up from repeating β-D-glucopyranose units (so-called anhydroglucose units (AGU)) that are closed through acetal functions between the OH group on C-4 and the C-1 carbon atom. Due to this linkage constraint and the β-positioning at the anomeric carbon, every unit is oriented at 180o with respect to its neighbours. The repeat unit of cellulose polymer, known as cellobiose, consists of two anhydroglucose rings (C6H10O5)n, linked by a β-1,4 glycosidic bond (Figure 1). The chain length expressed as the number of glucose unit in a polymer molecule (denoted as n) or also commonly regarded as degree of polymerization (DP) strongly depends on the source of the cellulose and treatment method to isolate the cellulose from plant sources. The DP values reported in the literature are in the range of hundreds to several tens of thousands [18]. For instance, cotton cellulose has a DP in the range of 800-10,000 which depends heavily on treatment, while the DP of wood pulp is found between 300 and 700 [14].
Each of the anhydroglucose units has three hydroxyl groups at the C-2, C-3 and C-6 positions; the alcohols at positions C-2 and C-3 are secondary alcohols while the alcohol at the C-6 position is a primary alcohol [19]. In general, these hydroxyl groups are accessible for chemical reaction. Furthermore, each cellulose chain has two chemically different ends: one end with a closed ring structure is known as the non-reducing end, while the opposite end has a free anomeric carbon atom of hemiacetal nature in equilibrium with an aldehyde, the so-called terminal reducing end.
As mentioned above, the chemical reactivity of cellulose is due to the presence of three hydroxyl groups in the AGU. The presence of these hydroxyl groups also creates an extensive hydrogen bonding network that makes cellulose insoluble in most aqueous and organic solvents. Thus, in the case of cellulose homogeneous reactions, this hydrogen bonding network must be disrupted to allow dissolution of the cellulose. In addition, the stability of the molecule is also governed by its sensitivity to hydrolytic attack at the glycosidic linkage between the repeat units, which would result in depolymerization.
Supramolecular structure
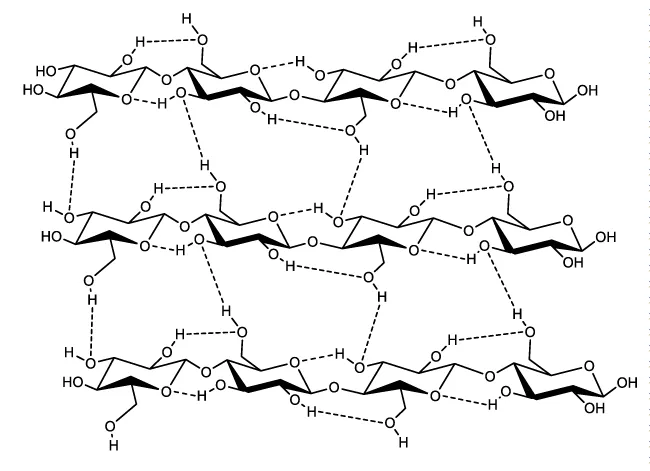
The intramolecular and intermolecular hydrogen bond network is formed between hydroxyl groups on one glucose unit with neighbouring glucose base units either within the same chain or from another chain (Figure 2). Intramolecular hydrogen bonds are formed as a result of interactions between O3-H and O-5’ of adjacent units as well as between O2-H and O-6’ of neighbouring glucose units. These intramolecular hydrogen bonds prohibit free rotation of the glucose rings around their linking glycosidic bonds and this contributes to chain stiffening and the stability of the two-fold crystalline cellulose conformation [1]. The intermolecular hydrogen bonds on the other hand are believed to exist between O-6-H and O-3 of neighbouring chains. These intermolecular hydrogen bonds contribute to interchain cohesion and packing.
Morphology
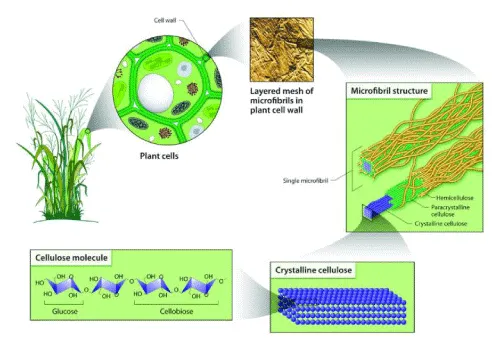
Cellulose exists naturally as assemblies of individual cellulose chains forming fibres (Figure 3). During biosynthesis in plants, cellulose is synthesized as individual molecules by specific enzymatic terminal complexes which then associate into chains. The multiple chains of cellulose then self-assemble into larger units of protofibrils [20], which subsequently pack into microfibrils through van de Waals and inter- and intramolecular hydrogen bonding and finally into cellulose fibres [6].
Source of cellulose
Cellulose occurs mostly as part of lignocellulosic material or semi-pure fibres. Lignocellulosic materials refer to plants that consist of cellulose, hemicellulose and lignin. Cellulose obtained from these sources needs to be isolated through pulping to remove unwanted lignin, hemicellulose and other extractives such as pectins, waxes, mineral matters, and proteins. Wood, which contains approximately 40% cellulose, has remained the major source of cellulose in the form of wood pulp, accounting for 90% of global pulp production. This is largely due to the availability of wood supply from forests or plantations in major parts of the world. Non-wood materials like flax, jute and kenaf, which possess a slightly lower cellulose content of about 30%, can only contribute 5-10% of pulp worldwide due to (among other constraints) its seasonal supply and availability when compared to wood plants.
Of all the plant fibres, cotton is however an exception as it is readily available as near pure fibres (95-99% cellulose in its original form). However, cotton also requires further processing and purification to remove the remaining non-cellulosic materials such as proteins, organic acids, and inorganic salts [21].
Interestingly, apart from the plant kingdom, cellulose can also be derived from non-plant materials such as algae, sea creatures, bacteria and fungi [6]. Driven by the effort to obtain pure cellulose, several studies have been conducted to produce cellulose from bacteria, in particular from Acetobacter xylinum. Despite its appealing properties (higher purity and crystallinity than plant cellulose), the associated cost involved in the production needs to be taken into account if mass production is needed. The widespread availability and continuous supply of cellulose from plant fibres seems to offer the cheaper option.
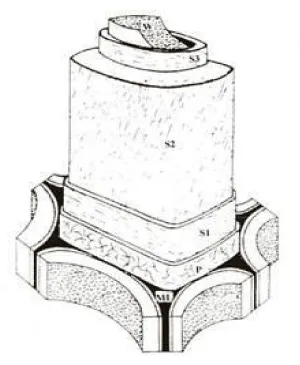
In plants, cellulose is produced in the cell walls. Cell walls in woody plants comprise several layers namely middle lamella (ML), primary wall (P), secondary wall (S1, S2 and S3) and warty layer (W) (Figure 4). Middle lamella (ML) being the outer layer of cell walls acts as a glue that binds neighbouring cells. Having a thickness of 0.25 µm, this is where pectins and proteinaceous materials exist. The primary wall (P) has a thickness less than 0.5 µm and consists of 50% cellulose along with pectin, protein and waxy material. The exact content varies depending on species and age. The secondary wall is further divided into S1, S2 and S3 layers having thickness between 0.1 to 5 µm and mainly functions as a support. Most cellulose is found in the secondary wall, particularly in the S2 layer, which is the thickest one. The three layers are distinguishable due to their different microfibril helix angle (varies between 5-90o) with respect to the fibre axis. Notably, the specific properties displayed by the S2 layer influence the fibre stiffness [21]. The microfibril angles found in this layer are usually 5-30o relative to the fibre axis.
Nanocellulose
Nanoparticles are defined as particles with at least one dimension between 1 and 100 nm. The properties of materials at this scale are different from their corresponding bulk material due to the high surface area to bulk ratio, so that new products can be created by selective manipulation [22]. For thousands of years, forests have provided a mass abundance of lignocellulosic material resources that can be exploited for various applications. Indeed, forest products have long been available in the form of timbers, wood products and many other functional materials. Along with the increasing demand for high performance material, traditional forest products need to expand towards the creation of highly engineered materials to find broader and more advanced applications. The quest for such material can be realized by looking into the hierarchical structure of plants namely the cellulose microfibrils, the nanosized building blocks functioning as the reinforcement unit that can be exploited to provide unique mechanical strength, functionality and flexibility [20].
There are generally three types of nanocelluloses: cellulose nanocrystals, cellulose nanofibrils and bacterial cellulose. The first two types of nanocellulose can be prepared using top down approaches. Cellulose nanocrystals (CNC) are commonly prepared via an acid hydrolysis of pure cellulose mostly using inorganic acids. Cellulose nanofibrils (CNF) are prepared via mechanical disintegration using high shear forces and potentially in combination with either chemical or enzymatic pre-treatment to reduce energy consumption. In the literature, many fibrillation methods have been presented to produce cellulose nanofibers such as the use of a high pressure homogenizer, an ultra-fine grinder, or a microfluidizer. Bacterial cellulose on the other hand is prepared via bottom-up approach i.e by the use of cellulose producing bacteria such as Acetobacter xylinum. The process is an extracellular in which glucan chain is first extruded from the cellulose-synthesizing complex, leaves the pores at the bacterium surface and then assembled to form microfibrils.
Looking into the literature, there are many terminologies that have been used to describe the crystalline rod-like nanoparticles. Examples include CNC, whiskers, nanoparticles, nanocrystalline cellulose, linters, and even microcrystallites. Similarly nanofibrillated cellulose has also gone through many names as nanofibrillated cellulose, cellulose nanofibrils and microfibrillated cellulose. Cellulose nanocrystals and cellulose nanofibrils will however be used throughout this paper. However, an attempt is in the pipeline to standardize the terminologies through the establishment of Standard Terms and Their Definition for Cellulose Nanomaterials, led by The Technological Association of the Pulp and Paper Industry (TAPPI), the leading association for the worldwide pulp and paper, packaging and converting industries [20].
The first few reported work on CNC by Nickerson and Habrle and also Rånby described the production of a colloidal cellulose suspension from different types of celluloses by controlled acid hydrolysis [23,24]. Inspired by the outstanding mechanical performance of composites incorporating CNC, CNC have come to the forefront at the end of the 1990s through various studies using them in nanocomposites as reinforcing material [25,26]. Since then, investigation on the use of CNC for nanocomposites and other applications has increased and has become an interest to many researchers not only by virtue of their biodegradability, availability, low cost, biocompatibility and easy chemical modification but also due to their nanoscale dimension, high surface area, high specific strength and modulus, and unique optical properties [26-31].
A study pioneered by Marchessault [32] found that colloidal CNC also displayed chiral nematic liquid crystalline properties which added another dimension to the CNC field of study. This field is currently receiving a large amount of attention to create photonic materials through CNC structuring [33,34].
CNC have been used as a template or support material to control the size, structure and organization of inorganic materials such as silica, titania, gold, silver and carbon-based materials [3]. Metal nanoparticles have received a growing interest because of their distinct electronic properties, high surface area which leads to increased catalytic performance. However, the unsupported metal nanoparticles are easily aggregated in the reaction systems hence leading to a lower catalytic performance. It is imperative to prevent this condition by depositing the metal nanoparticles on solid matrices such as metal oxides, polymers or fibres which results in reduction of total surface area. The catalytic performance of gold nanoparticles in various solid support in different reactions have been reported [35-39].
Nanofibrillated cellulose preparation from wood pulp was pioneered by Turbak et al. [40] using a high pressure homogenizer. Besides that, other defibrillation methods have been also used to produce CNF such as the use of grinder, sonicator, electrospinning machine and cryocrushing method using liquid nitrogen (will be described in later section).
Preparation
Cellulose nanocrystals
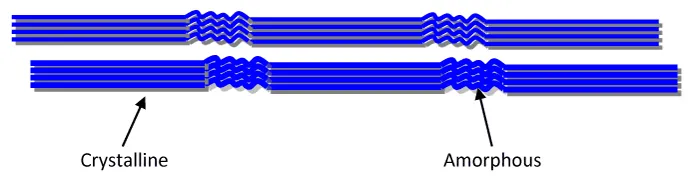
The cellulose microfibril structure is made up of crystalline and amorphous or disordered regions (Figure 5). Due to the reduced structure of the cellulose chain arrangement in the disordered region, it has a lower density and thus exhibits more free volume than the crystalline region [1]. Therefore, the acid preferably hydrolyses the disordered amorphous region leaving the crystalline region largely intact due to their tight packing [41]. With correct timing of the hydrolysis, rod-like CNC are produced as individualized particles.
CNC are isolated from native cellulose mostly via an acid hydrolysis. Enzymatic hydrolysis using cellulases [42,43], TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl)-mediated oxidation [44], and ionic liquids have also been reported to prepare cellulosic nanoparticles [45]. Recently, a new method using a gas-phase hydrochloric acid hydrolysis at room temperature for 30 min was patented [46]. In this method however, after hydrolysis, the ensuing cellulose still needs to be mechanically treated to finally obtain the desired nanocrystals.
During acid hydrolysis, a rapid decrease in the degree of polymerisation of cellulose occurs, until it levels off [47]. The point at which the depolymerization stabilizes is known as the level-off degree of polymerization (LODP). The reason for the initial reduction of DP is proposed to be due to rapid hydrolysis of amorphous regions because of a fringed micelle-like structure of microfibrils where amorphous and crystalline regions are located [48].
CNC have been prepared from various sources of cellulose fibres under different hydrolysis conditions ranging from wood pulp, bacterial cellulose, tunicin, non-wood plants, wood and agricultural residues [20,49]. There are several factors that have an effect on the resulting nanocrystal properties such as acid concentration, acid type, reaction time and temperature [50,51,52]. Mild conditions are essential to prevent the complete hydrolysis to glucose [49]. The variations in concentration, time and temperature were demonstrated to have the most influence on the morphology of CNC. Early work by Revol et al. reported that the hydrolysis worked best when the concentration of sulfuric acid is between 60-70% where 65% being the most common [53]. The optimization of hydrolysis conditions was carried out by Dong in which a hydrolysis at 64% concentration, 45oC for 1 hour gave an ivory white suspension [54]. Increasing the hydrolysis time shortened the nanocrystals whereas an increased temperature resulted in cellulose conversion to glucose [54]. In the beginning of the hydrolysis, the acid diffused preferentially into the non-crystalline region and hydrolysed the accessible glycosidic bonds. Once these more easily accessible glycosidic bonds in the fibres were hydrolysed, further reaction occurred much slowly at the reducing end and at the surface of the residual crystalline regions [54]. Work by Bondenson et al. observed shorter CNC and an increase in surface charge as a result of longer hydrolysis time [55]. A prolonged hydrolysis time and acid concentration also led to the reduction in length of CNC [56]. The same observation was also reported when increasing the hydrolysis temperature up to 72oC [57].
Although many strong acids have been attempted to hydrolyse cellulose, sulfuric and hydrochloric acid [3,4,58] have been studied the most. The use of sulfuric acid for the hydrolysis results in the introduction of sulfate esters through esterification of sulfuric acid with hydroxyl groups on the surface of cellulose. The negatively charged sulfate ester groups on the surface lead to the stabilization of the suspension due the electrostatic repulsion created between nanocrystals. Other examples of acids used in CNC production are hydrobromic, phosphoric [59-62] and nitric acid [63]. CNC prepared using hydrochloric acid result in aqueous dispersions with limited stability, most probably due to the limited formation of negative charges on the CNC surface. This was evident from the work by Araki [50] which showed that H2SO4-hydrolysed CNC from bleached wood pulp had a higher surface charge than those obtained from hydrochloric acid hydrolysis. Upon completion of hydrolysis, the resulting suspension of hydrolysed cellulose is subjected to dilution with water, successive centrifugation, dialysis, sonication and filtration.
Cellulose nanofibrils
Preparation of CNF is rather straightforward since it involves the use of refining machine to produce fibrils with nanosized dimensions. The main important issue that needs to be addressed when preparing CNF is how to minimise the use of energy. In the early work of CNF, the high energy consumption limited the commercialization of CNF due to the linked cost. Therefore, there are different pre-treatments that have since been developed to reduce the required mechanical disintegration energy such as chemical pre-treatment via carboxymethylation and TEMPO-mediated oxidation or enzymatic pre-treatment using cellulase to reduce energy consumption [64].
In the initial work describing CNF production, a laboratory Gaulin type milk high pressure homogenizer operating at 55 MPa was used [40]. The diluted pulp suspensions were passed through the homogenizer slits for repeated cycles. In the process, the slurries were subjected to high pressure and forced through a valve that opened and closed successively leading to fibrillation of the cellulosic fibres. After a specific number of passes (usually 10) through the homogenizer, the cellulose suspension turned translucent and more viscous. Besides using a homogenizer, a microfluidizer can also be used to produce CNF (Microfluidics Inc., USA). The process involves subjecting the cellulose suspension into z-shaped interaction chambers where high shear forces are generated leading to the mechanical disintegration of the fibers. This process is repeated a few times to improve the degree of fibrillation.
High intensity ultrasonication has also been reported for CNF production. The hydrodynamic forces from the ultrasound equipment lead to defibrillation of cellulosic fibres. Factors that affected fibrillation via sonication include power, temperature, time and consistency of the suspension [30].
Electrospinning is another method to prepare nanofibers that involves injecting a polymer solution to a high voltage thereby producing nanoscale fibres at the collector [65]. In order to produce electrospun fibres, the most important element is to find a suitable solvent for the polymer. As cellulose is not soluble in most common solvents, one alternative is to prepare cellulose derivatives such as cellulose acetate, methylcellulose and carboxymethyl cellulose sodium salt [66].
Morphology
The morphology of nanocellulose is distinctive for CNC and CNF. Table 1 shows the reported morphology of nanocellulose obtained from various sources and by different preparation methods.
The highly ordered CNCs produced by acid hydrolysis have different dimensions depending on the origin of cellulose and process conditions as mentioned previously. The dimensions of CNC are generally determined using scanning electron microscopy (SEM), atomic force microscopy (AFM) and/or transmission electron microscopy (TEM). SEM is usually used to get a general overview but is incapable of more detailed morphology. AFM can scan materials up to nanometers but usually overestimate the width due to tip-broadening effect. The width of nanocrystals is typically a few nanometers whereas the length is in the hundreds of nanometers.
For instance, CNC from cotton generally display 100-300 nm in length and 5-10 nm in width, tunicates produced CNC with 1000-2000 nm in length and 10-20 nm in width whereas wood can produce 100-200 nm in length and 3-5 nm in width CNC [1]. Thus, the aspect ratio of those materials calculated as the length divided by the width (l/w) are reported to be in the range of 10-70. The variations are due to the starting materials to a large extent and are also partially attributed to the acid hydrolysis conditions to which the materials are subjected to [6].
Properties of nanocellulose
Nanocellulose is a unique material due to its interesting inherent properties; high mechanical strength, high surface area, interesting optical and rheological properties and ease of surface modification. Theoretically, crystalline regions of cellulose have tensile strength in the range between 50-100 GPa [2]. This is stronger than E-glass as well as Kevlar both having 3,400 MPa and 3000 MPa respectively [49]. The strongly interacting surface hydroxyl group on nanocellulose contributes to self association. This property makes nanocellulose a good reinforcing material in composite matricesh for the formation of load-bearing percolating architectures within a host polymer matrix. Stress transfer is due to hydrogen-bonding among the nanowhiskers. Besides being stronger than glass, cellulose nanocrystals posses birefrigent behaviour that can exhibit liquid crystalline optical property. This liquid crystalline nature can find applications in security paper. Nanocellulose also shows shear thinning and anomalous manner which are useful for rheology property.
Applications of nanocellulose
A recent publication has covered the potential applications of nanocellulose based on interviews and literature survey [67,68]. Table 2 shows the identified applications of nanocellulose based on volumes of existing applications as well as new ones. The largest volumes are expected to cover the areas of cement, paper packaging, and automobile parts. Applications such as aerospace materials, paints and insulation are rather low volume applications. Niche applications such as sensors and electronics may not be in demand yet as more research is still needed to develop these further.
Conclusion
Nanocellulose has many to offer due to its being renewable and biodegradable as well as its many interesting and unique characteristics. Nanocellulose has many potential applications in many areas. This includes from basic products such as packaging, cements, paper products, automotive as well as niche products such as flexible electronic, sensor and many others. Turning wood into nanocellulose could create new wealth and revenue for the country.
- Souza DE, Lima MM, Borsali R (2004) Rodlike cellulose microcrystals: Structure, properties, and applications. Macromolecular Rapid Communications 25: 771-787. Link: https://goo.gl/BqeXM2
- Eichhorn SJ, Dufresne A, Aranguren M (2010) Review: current international research into cellulose nanofibres and nanocomposites. Journal of Materials Science 45: 1-33.
- Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chemical Society Reviews 43: 1519-1542. Link: https://goo.gl/bNDu36
- Klemm D, Kramer F, Moritz S (2011) Nanocelluloses: A New Family of Nature-Based Materials. Angewandte Chemie International Edition 50: 5438-5466. Link: https://goo.gl/vw5mo8
- Payen A (1838) Mémoire sur la composition du tissu propre des plantes et du ligneux Comptes Rendus 7: 1052-1056.
- Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chemical Reviews 110: 3479-3500. Link: https://goo.gl/3yDLnP
- Staudinger H (1926) The chemistry of high molecular organic substances according to Kekule's structure theory. Berichte Der Deutschen Chemischen Gesellschaft 59: 3019-3043.
- Fengel D, Wegener G (1984) Wood: Chemistry, Ultrastructure, Reactions
- Hon DNS, Shiraishi N (1991) Wood and cellulosic chemistry. Wood and Cellulosic Chemistry.
- O'sullivan AC (1997) Cellulose: The structure slowly unravels. Cellulose 4: 173-207.
- Atalla RH (1999) Celluloses. Comprehensive Natural Products Chemistry 3: 529-598.
- Atalla RH, Vanderhart DL (1999) The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nuclear Magnetic Resonance 15: 1-19. Link: https://goo.gl/3LdjtX
- Atalla RH (2000) The individual structures of native cellulosesLenzinger Berichte 79: 5-14.
- Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angewandte Chemie International Edition 44: 3358-3393. Link: https://goo.gl/xKpKJb
- Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: Fascinating biopolymer and sustainable raw material. Angewandte Chemie - International Edition 44: 3358-3393. Link: https://goo.gl/BW24Pj
- Atalla RH, Brady JW, Matthews JF, Ding SY (2008) Structure of plant cell wall celluloses, Blackwell.
- Atalla RH, Brady JW, Matthews JF, Ding SY (2008) Structure of plant cell wall celluloses, Blackwell.
- Serge P, Karim M (2004) Conformations, Structures, and Morphologies of Celluloses. Polysaccharides: Structural Diversity and Functional Versatility. S. Dumitriu, CRC Press. Link: https://goo.gl/hSdh36
- Klemm D, Phillip B, Heinze T, Heinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry. Fundamentals and analytical methods. Link: https://goo.gl/A8wUbB
- Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: Structure, properties and nanocomposites. Chemical Society Reviews 40: 3941-3994. Link: https://goo.gl/ZCDjXA
- Sjostrom E (1993) Wood chemistry: fundamentals and applications Academic Press.
- Kamel S (2007) Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polymer Letters 1: 546-575. Link: https://goo.gl/63BzQi
- Nickerson, RF, Habrle JA (1947) Cellulose Intercrystalline Structure. Industrial & Engineering Chemistry 39: 1507-1512.
- Rånby BG (1951) Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discussions of the Faraday Society 11: 158-164. Link: https://goo.gl/r96DnP
- Favier V, Chanzy H, Cavaillé JY (1995) Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 28: 6365-6367. Link: https://goo.gl/u6RNim
- Garcia DE, Rodriguez NL, Thielemans W, Dufresne A (2006) Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites. Cellulose 13: 261-270. Link: https://goo.gl/tdnqsm
- Azizi Samir MAS, Chazeau L, Alloin F, Cavaillé JY, Dufresne A, et al. (2005) POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Electrochimica Acta 50: 3897-3903. Link: https://goo.gl/LFxMff
- Dong S, Roman M (2007) Fluorescently labeled cellulose nanocrystals for bioimaging applications. Journal of the American Chemical Society 129: 13810-13811. Link: https://goo.gl/wNc3HM
- Jiang L, Morelius E, Zhang J, Wolcott M, Holbery J (2008) Study of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites prepared by solution casting and melt processing. Journal of Composite Materials 42: 2629-2645. Link: https://goo.gl/tBCHxR
- Wang S, Cheng Q (2009) A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, part 1: Process optimization. Journal of Applied Polymer Science 113: 1270-1275. Link: https://goo.gl/84vWFi
- Peng BL, Dhar N, Liu HL, Tam KC (2011) Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. The Canadian Journal of Chemical Engineering 89: 1191-1206. Link: https://goo.gl/Jt4oHK
- Marchessault RH, Morehead FF, Walter NM (1959) Liquid crystal systems from fibrillar polysaccharides. Nature 184: 632-633. Link: https://goo.gl/Q2iior
- Lagerwall JPF, Schütz C, Salajkova M (2014) Cellulose nanocrystal-based materials: from liquid crystal self-assembly and glass formation to multifunctional thin films. Npg Asia Materials 6: e80. Link: https://goo.gl/L1qnst
- Gençer A, Schütz C, Thielemans W (2017) Influence of the Particle Concentration and Marangoni Flow on the Formation of Cellulose Nanocrystal Films. Langmuir 33: 228-234. Link: https://goo.gl/FsMRhH
- Panigrahi S, Basu S, Praharaj S (2007) Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process. Journal of Physical Chemistry C 111: 4596-4605. Link: https://goo.gl/Cm5mnB
- Wang Y, Wei G, Zhang W (2007) Responsive catalysis of thermoresponsive micelle-supported gold nanoparticles. Journal of Molecular Catalysis A: Chemical 266: 233-238. Link: https://goo.gl/jaJo6D
- Kuroda K, Ishida T, Haruta M (2009) Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. Journal of Molecular Catalysis A: Chemical 298: 7-11. Link: https://goo.gl/5aZ6q4
- Lam E, Hrapovic S, Majid E, Chong JH, Luong JHT (2012) Catalysis using gold nanoparticles decorated on nanocrystalline cellulose. Nanoscale 4: 997-1002. Link: https://goo.gl/MEqqLy
- Lam E, Male KB, Chong JH, Leung ACW, Luong JHT (2012) Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends in Biotechnology 30: 283-290. Link: https://goo.gl/X9PJSy
- Turbak AF, Snyder FW, Sandberg KR (1983). Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci.: Appl. Polym. Symp. 37: 815-827.
- Anglès MN, Dufresne A (2001) Plasticized starch/tunicin whiskers nanocomposite materials. 2: Mechanical behavior. Macromolecules 34: 2921-2931. Link: https://goo.gl/uhRWTs
- Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. European Polymer Journal 43: 3434-3441. Link: https://goo.gl/mwUVXj
- Siqueira G, Tapin-Lingua S, Bras J, Da Silva Perez D, Dufresne A (2010) Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 17: 1147-1158. Link: https://goo.gl/4ZDn68
- Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8: 2485-2491. Link: https://goo.gl/n5UvJi
- Man Z, Muhammad N, Sarwono A, Bustam MA, Kumar MV, et al. (2011) Preparation of Cellulose Nanocrystals Using an Ionic Liquid. Journal of Polymers and the Environment 19: 726-731. Link: https://goo.gl/uCGjbC
- Nickerson RF, Habrle JA (1947) Cellulose intercrystalline structure. Study by hydrolytic methods. Ind. Eng. Chem. 39: 1507-1512.
- Nickerson, RF, Habrle JA (1947) Cellulose Intercrystalline Structure. Industrial & Engineering Chemistry 39: 1507-1512.
- Araki J (2013) Electrostatic or steric?-preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 9: 4125-4141. Link: https://goo.gl/EvN6vR
- Dufresne A (2012) Nanocellulose, From Nature to High Performance Tailored Materials, Walter de Gruyter GmbH.
- Araki J, Wada M, Kuga S, Okano T (1998) Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids and Surfaces A: Physicochemical and Engineering Aspects 142: 75-82. Link: https://goo.gl/7QYZs3
- Araki J, Wada M, Kuga S, Okano T (1999) Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. Journal of Wood Science 45: 258-261. Link: https://goo.gl/kYeUCo
- Bai W, Holbery J, Li K (2009) A technique for production of nanocrystalline cellulose with a narrow size distribution. Cellulose 16: 455-465. Link: https://goo.gl/2uxwMw
- Revol JF, Godbout L, Dong XM, Gray DG, Chanzy H, et al. (1994) Chiral nematic suspensions of cellulose crystallites; phase separation and magnetic field orientation. Liquid Crystals 16: 127-134. Link: https://goo.gl/Ayjftu
- Dong XM, Revol JF, Gray DG (1998) Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5: 19-32. Link: https://goo.gl/mJ8QSL
- Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13: 171-180. Link: https://goo.gl/mMXmeo
- Beck-Candanedo S, Roman M, Gray DG (2005) Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 6: 1048-1054. Link: https://goo.gl/N8T9Wi
- Azizi Samir MAS, Alloin F, Sanchez JY, El Kissi N, Dufresne A (2004) Preparation of cellulose whiskers reinforced nanocomposites from an organic medium suspension. Macromolecules 37: 1386-1393. Link: https://goo.gl/x6fS9f
- Yu H, Qin Z, Liang B, Liu N, Zhou Z, et al. (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. Journal of Materials Chemistry A 1: 3938-3944. Link: https://goo.gl/4cF5Nm
- Carpenter BL, Feese E, Sadeghifar H, Argyropoulos DS & Ghiladi RA (2012) Porphyrin-cellulose nanocrystals: A photobactericidal material that exhibits broad spectrum antimicrobial activity. Photochemistry and Photobiology 88: 527-536. Link: https://goo.gl/LhMCGf
- Feese E, Sadeghifar H, Gracz HS, Argyropoulos DS, Ghiladi RA (2011) Photobactericidal porphyrin-cellulose nanocrystals: Synthesis, characterization, and antimicrobial properties. Biomacromolecules 12: 3528-3539. Link: https://goo.gl/2MwWQf
- Koshizawa T (1960) Degradation of wood cellulose and cotton linters in phosphoric acid. Kami Pa Gikyoshi 14: 455. Link: https://goo.gl/QyTRsj
- Camarero Espinosa S, Kuhnt T, Foster EJ, Weder C (2013) Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 14: 1223-1230. Link: https://goo.gl/RPNH8r
- Liu D, Zhong T, Chang PR, Li K, Wu Q (2010) Starch composites reinforced by bamboo cellulosic crystals. Bioresource Technology 101: 2529-2536. Link: https://goo.gl/CKrwHN
- Dufresne A (2006) Comparing the mechanical properties of high performances polymer nanocomposites from biological sources. Journal of Nanoscience and Nanotechnology 6: 322-330. Link: https://goo.gl/WdJY4t
- Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology 63: 2223-2253. Link: https://goo.gl/MytWLn
- Liu H, Hsieh Y (2002) Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. Journal of Polymer Science Part B: Polymer Physics 40: 2119-2129. Link: https://goo.gl/RYbWQP
- Shatkin JA, Wegner T, Bilek EM, Cowie J (2014) Market projections of cellulose nanomaterial-enabled products-Part 1: Applications. Tappi Journal 13: 9-16. Link: https://goo.gl/PWiHq3
- Chen G, Dufresne A, Huang J, Chang PR (2009) A Novel Thermoformable Bionanocomposite Based on Cellulose Nanocrystal-graft-Poly(ε-caprolactone). Macromolecular Materials and Engineering 294: 59-67. Link: https://goo.gl/uHqs59
- Jasmani L, Adnan S (2017) Preparation and characterization of nanocrystalline cellulose from Acacia mangium and its reinforcement potential. Carbohydrate Polymers 161: 166-171. Link: https://goo.gl/5GxVto
- Lu P, Hsieh YL (2012) Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydrate Polymers 87(1): 564-573. Link: https://goo.gl/PJu4MQ
- Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci.: Appl. Polym. Symp 37: 797-813.
- Taniguchi T, Okamura K (1998) New films produced from microfibrillated natural fibres. Polymer International 47: 291-294. Link: https://goo.gl/X2RTP2
- Jonoobi M, Harun J, Mathew AP, Oksman K (2010) Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Composites Science and Technology 70: 1742-1747. Link: https://goo.gl/fvwUqB
- Roberts JC (1996) The chemistry of paper [electronic resource J.C. Roberts, Royal Society of Chemistry.
- U.S. DEPARTMENT OF ENERGY OOS (2018) 'Genomics: GTL Roadmap.' Retrieved.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
