International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Validation of sterilization of Lucilia cuprina larvae using raw honey through scanning electron microscope
Nur Afrina MH1*, Santhana Raj L1, Nur Fateha R1, Wan Nur Alia WZ2, Kamesh R3 and Nazni WA2
2Entomology Unit, Institute for Medical Research, Ministry of Health Malaysia, Jalan Pahang, 50588 Kuala Lumpur, Malaysia
3Environmental Health Research Centre, Institute for Medical Research, Ministry of Health Malaysia, Jalan Pahang, 50588 Kuala Lumpur, Malaysia
Cite this as
Nur Afrina MH, Santhana Raj L, Nur Fateha R, Wan Nur Alia WZ, Kamesh R, et al. (2019) Validation of sterilization of Lucilia cuprina larvae using raw honey through scanning electron microscope. Int J Nanomater Nanotechnol Nanomed 5(2): 012-015. DOI: 10.17352/2455-3492.000030Background: In the application of Maggot Debridement Therapy (MDT), sterilized maggots (Lucilia cuprina) by standard chemicals were used. Recent potential demands for using a procedure with all-natural products, raw honey has been proposed as a natural sterilization technique to replace the chemical products.
Methods: Lucilia cuprina collected from a laboratory colony were divided into treated group (sterilized) and control group (non-sterilized) to undergo sterility testing with honey dilution. Scanning electron microscope (SEM) was used to examine and ascertain the sterility of honey dilution. SEM provided direct observation of microorganism’s presence on external surface L. cuprina after sterilization by disinfectants of raw honey (tualang honey).
Results: Treated group showed the absence of microorganism on the surface body segment of the larvae. It reflected in different magnifications in which treated maggots appeared to be smooth and clear of any external contaminants. The untreated maggots exhibited clusters of microorganisms on the external body surface.
Conclusion: This study confirmed the complete sterilization of maggots using raw honey as the antibacterial agent using SEM due to its disinfection efficacy in destroying microorganisms in L.cuprina.
Introduction
Maggot Debridement Therapy (MDT) became an emerging trend as a natural remedy that can heal wounds. For the application of MDT, sterilized blow fly (Lucilia cuprina), commonly called maggots, were used. The MDT product used sodium hypochlorite and formaldehyde as the main disinfectants of the maggots. This had been validated by using a Scanning Electron Microscopic (SEM) that identifies the absence of bacteria on maggots, in which this process has been approved by the Ministry of Health of Malaysia as a safe procedure to use [1].
Due to increasing demand by potential clients who want to use a procedure with all natural products, raw honey has been proposed as a natural sterilization technique to replace the chemical products. Raw honey was discovered as an effective therapeutic agent because it contains potent antimicrobial action against a broad spectrum of bacteria and fungi [2]. Jenkins (2012) noted that all types of honey have high hydrogen peroxide, sugar content and acidity, which prevents microbial growth [3]. Glucose and fructose oxidization will also produce hydrogen peroxide, which can inhibit and destroy bacteria cell walls. When honey is diluted with water, the enzymes create a small amount of hydrogen peroxide that gives an exothermic effect of being bactericidal [4].
Although it has not been proven that honey actually sterilized maggots, this study has the potential to be explored further. This helps us in exploring extensively the usage of raw honey as an antibacterial agent. This finding could provide avenue for further research on honey, in sterilizing the maggots as chemical usage can be reduced. This study also evaluated the process of sterilization of maggots using raw honey, validated with an SEM.
Materials and Methods
Raw honey
Pure raw honey (tualang honey) was obtained from Federal Agriculture Marketing Authority (FAMA), Malaysia, which was collected from Koompassia excelsa (tualang tree). For the sterilization process, the honey was diluted with distilled water (1:10). All the assays were performed in laminar flow.
Maggots colony
The colonies of L.cuprina used in this study were maintained under 27ºC-29ºC with relative humidity of about 70% in the insectarium of Institute for Medical Research, Kuala Lumpur. The lab technician fed the flies with pieces of cow liver to provide protein source to induce oviposition. The oviposited flies then laid eggs and converted into maggots (3rd instar larvae) within an average of 4 days.
Preparation for the sterility of maggots
L. cuprina maggots (3rd instar larvae) were removed from the cow liver and divided into treated and control groups. The raw honey was diluted with distilled water prior to the sterilization process. The dilution prepared is one part of raw honey in nine parts of sterile distilled water in the ratio of 1:10. The treated group was surface sterilized with honey dilution by stirring for 5 minutes then washed with sterile distilled water. This process was repeated three times. The untreated group was only washed in sterile distilled water.
Preparation of maggots for SEM
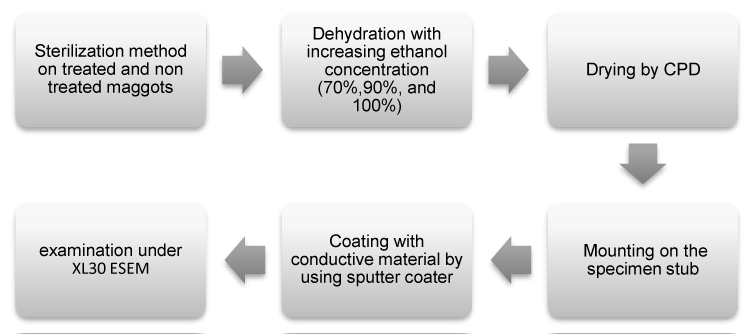
Sixty maggots were examined with 30 in each group under SEM in three magnifications; x300, x1500, x6500. The treated and untreated maggots went through SEM processing procedure using increasing concentrations of ethanol 70%, 90% and 100% (3 times) in 5 minutes respectively for dehydration. Subsequently the maggot samples were dried by using critical point dryer (CPD). Therefore, the ethanol is replaced by CO2 fluid under 1072 psi pressure (10°C). After complete exchange of fluids, the maggot samples were heated up to 40°C. By heating up, the CO2 fluid would be phased into CO2 gas. Then the CO2 gas were gently released from the CPD device until atmospheric pressure is obtained. The dried material was mounted on holders or stubs, sputter coated with a thin layer of conductive material (42nm gold thickness) and then ready to be viewed by SEM (Philips XL-30 ESEM, IMR, Malaysia). The flow chart of the SEM processing is shown in Figure 1.
Results
The treated maggot’s group showed absence of microorganisms on the surface body segment of the larvae (Figure 2). The third instar larvae consisted of three main components (cephalic, thoracic, and segmented abdominal) and band of spines between the segments. We focused on the abdominal area because it consisted of eight segments. It reflected in different magnifications in which treated maggots appeared to be smooth and clear of any external contaminants (Figure 2 A-C).
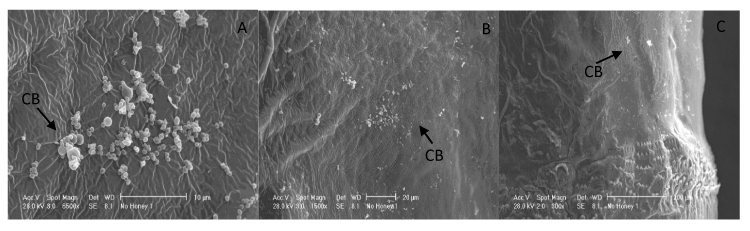
On the other hand, the untreated maggots exhibited clusters of microorganisms on the external surface with three different SEM magnifications (Figure 3). The microorganism species were not identified or differentiated, but however, by observing the magnifications of the electron microscopic images, we noticed that they were more Cocci-like and less bacilli-like. This was because Cocci bacteria were more commensal to the environment compared to Bacillus bacteria.
Discussion
Treated maggots with raw honey (tualang honey) showed a sign of complete sterilization as judged by absence of bacteria or any other microorganisms. However, untreated maggots showed presence of bacteria and other microorganisms on the external surface of maggot samples. The bacteria and microorganisms found were categorized as contaminants. Although we never carry out blood agar culture test to emphasize the complete sterilization test, observation done by SEM showed clear surface on the maggots as sterilization can be accomplished by diluted raw honey.
This preliminary study determined the efficacy of raw honey to sterilize maggots. From previous studies, several species of bacteria isolation had been demonstrated from L. cuprina such as pathogenic E. coli, which causes diarrhea [5]; and P. mirabilis, which causes urinary tract infection and systemic inflammatory response syndrome [6]. It is particularly interesting that we also detected Cocci-like bacteria that was often regarded as contaminants.
This study provided SEM as a validation tool to confirm the sterility of the maggots produced after sterilization before MDT application. Our results indicated that sterility of maggots was successfully achieved after sterilization with honey based on SEM observation.
Sterilization process used in MDT is a rigorous process that results in sterility but produces a high number of eggs after the sterilization process [7]. However, in this study only 3rd instar larvae were used as a proxy to determine the efficacy of raw honey in sterilizing the larvae surfaces. Baer (1931) reported that it was necessary to use sterile maggots on patients [8]. In his early experiment, non-sterile maggots were used as MDT debriding agent; however, patients subsequently developed secondary infection such as tetanus.
In addition to Baer (1931), Nuesh et al., (2002) also concluded that it was essential to use only sterile maggots on patients. Recent studies showed that the best sterilization method is to sterilize the eggs as compared to sterilization of maggots, and this observation was in line with Baer’s finding [8]. In this study, we used 3rd instar larvae as the proxy instead of eggs to get preliminary data in short period of time to ensure this study works effectively that could lead to further research.
For this study, raw tualang honey was selected as the disinfectant to sterilize L. cuprina surface without affecting its larval survival. The sterilization process was successful which was confirmed through SEM evaluation. In raw honey, the properties that contributed to antibacterial activity were hydrogen peroxide (H2O2), high osmolarity, and presence of phytochemical components (non-peroxide) [9].
H2O2 is produced in honey by oxidation of glucose by the presence of glucose enzyme in the honey. H2O2 reacts to the bacteria by affecting the outer membrane of the bacteria. By doing so, the bacteria will have shorter survival rate and do not multiply or do not show mitosis effect. Due to mitosis process, multiplication of bacteria will not happen. Directly, the bacteria will be killed and do not cause primary and secondary infections [10]. This proves that raw honey has the antibacterial effect and confirms our obtained results.
As mentioned above, H2O2 will puncture holes on the outer membrane of the bacteria which will cause differential osmotic pressure between the internal and external surface of the bacteria. Whereby, the external osmotic pressure will cause a blown-up effect that will rupture the outer membrane of the bacteria [11]. By the blow-up effect, antibacterial activity of raw honey will be more compressive.
Besides H2O2 and osmotic effect, other antibacterial activity in honey is the non-peroxidase pathway. In our study, the tualang honey that was collected in the jungle had variation of phytochemical compounds obtained by the bees from various plants in honey making. Alvarez-Suarez and colleagues (2014) studied that honey made by bees had phytochemical compounds that was shown to heal sore throats in patients [12]. This phytochemical component was attributed to hydrogen peroxide and high osmolarity. These effects helped our study to explain the antibacterial activity more complete and more compressively.
Various activities in honey will affect various bacteria it may contain. Tualang honey used in this study gave positive responses towards suppression of bacterial growth on the external surface of maggots. Besides its antimicrobial properties, honey clears infection in a number of ways, including boosting the immune system, having anti-inflammatory and antioxidant activities and stimulation of cell growth [10]. There are number of studies on honey's therapeutic properties, along with the rapidly increasing interest in research into natural health remedies and supplements, which has led to increasing interest in research into natural health remedies and supplements.
Conclusion
This study confirmed the complete sterilization of maggots using raw honey as the antibacterial agent by using SEM. This study suggests that raw honey can be used as a disinfectant to sterilize the blowfly maggots effectively and safely. Raw honey can be used therapeutically due to the characteristics of having higher H2O2 content, osmotic affects, and presence of phytochemical compounds. The SEM should always be part of the routine investigation of bacteria after sterilization in order to verify the safety of MDT. Nevertheless, we recommend more researches, specifically focused on the effect of honey compounds on the microorganisms, to support further findings.
We would like to thank the Director General of Health, Malaysia for his permission to publish this article, and Director of Institute for Medical Research (IMR), Kuala Lumpur for support. This research was supported by grants from SEAMEO-TropMed, 2018 through the research administration office of Diploma of Applied Parasitology and Entomology, IMR.
- Yeong YS, Nazni WA, Santana RL, Mohd Noor I, Lee HL et al. (2011) Scanning electron microscopic evaluation of the successful sterilization of Lucilia cuprina (Wiedemann) utilized in maggot debridement therapy (MDT). Trop Biomed 28: 325-332. Link: http://bit.ly/2C0nQ8q
- Mandal MD, Mandal S (2011) Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1: 154-160. Link: http://bit.ly/333NOU6
- Jenkins R, Cooper R (2012) Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 7: e45600. Link: http://bit.ly/36jJsdz
- Subrahmanyam M (2007) Topical application of honey for burn wound treatment - An Overview. Ann Burns Fire Disasters 20: 137–139. Link: http://bit.ly/2ps8VB5
- Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, et al. (2001) Practice guidelines for the management of infectious diarrhea. Clinical Infectious Diseases 32: 331-351. Link: http://bit.ly/2N3Glig
- Schaffer JN, Pearson MM (2015) Proteus mirabilis and Urinary Tract Infections. Microbiol Spectr 3. Link: http://bit.ly/2qaSEjP
- Nuesch R, Rahm G, Rudin W, Steffen I, Frei R, et al. (2002) Clustering of bloodstream infections during maggot debridement therapy using contaminated larvae of Protophormia terraenovae. Infection 30: 306-309. Link: http://bit.ly/325fjLH
- Baer WS (2011) The classic: The treatment of chronic osteomyelitis with the maggot (larva of the blow fly). Clin Orthop Relat Res 469: 920-944. Link: http://bit.ly/36iryrK
- Israili ZH (2014) Antimicrobial properties of honey. Am J Ther 21: 304-323. Link: http://bit.ly/34rdc6T
- Tan HT, Rahman RA, Gan SH, Halim AS, Hassan SA, et al. (2009) The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement Altern Med 9. Link: http://bit.ly/336kXi8
- Santhana Raj L, Hing HL, Baharudin O, Teh Hamidah Z, Aida Suhana R, et al. (2007) Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923. Trop Biomed 24: 105-109. Link: http://bit.ly/338s89K
- Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F (2014) The composition and biological activity of honey: A focus on manuka honey. Foods 3: 420-432. Link: http://bit.ly/2BZ6HvI

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
