Open Journal of Analytical and Bioanalytical Chemistry
Determination of Magnesium in Blood Serum by Using Carbon Paste Ion Selective Electrode Based on Multi-Walled Carbon Nanotubes and Nano Silicon
Hadi Gohari*
Cite this as
Gohari H (2016) Determination of Magnesium in Blood Serum by Using Carbon Paste Ion Selective Electrode Based on Multi-Walled Carbon Nanotubes and Nano Silicon. Open J Anal Bioanal Chem 1(1): 001-006. DOI: 10.17352/ojabc.000001The number of trace metallic elements of biological importance is increasing and a wide range of elements need to be analyzed in blood, urine and tissues. There is often a natural limitation of sample volume particularly in the analysis of blood and tissue digest samples. A new Mg2+Carbon paste ion selective electrode based on4,5-Bis (benzoylthio)-1,3-dithiole-2-thione (BZ2dmit) for determination of trace amount of Magnesium in Blood Serum was prepared. Using nanosilica and multi-walled carbon nanotube (MWCNT) in the composition of carbon paste electrodes causes improvement in their characterizations. The electrode composition of 3% MWCNT ,0.5% nanosilica ,25% paraffin oil, 68.5% graphite powder, 3%(BZ2dmit)..The proposed sensor shows a Nernstian response (29.9±0.7 mV decade-1) in the range of 1.0×10-9-1.0×10-2 M with a detection limit of 5.0 × 10-10 M. The response of the sensor is independent of pH in the range of 3.4 – 8.9, and its at least 28-week applicability life time, possesses the advantages of fast response time about 7s, The matched potential method (KMPM) was applied to assess the selectivity coefficients for different cations toward new prepared electrode. According to obtained results of this Mg2+nano-composite carbon paste sensor exhibited good selectivity with respect to a number of lanthanide and transition metal ions. The sensor was effectively used as an indicator electrode in the potentiometric titration of Mg (II) ions with EDTA.
Introduction
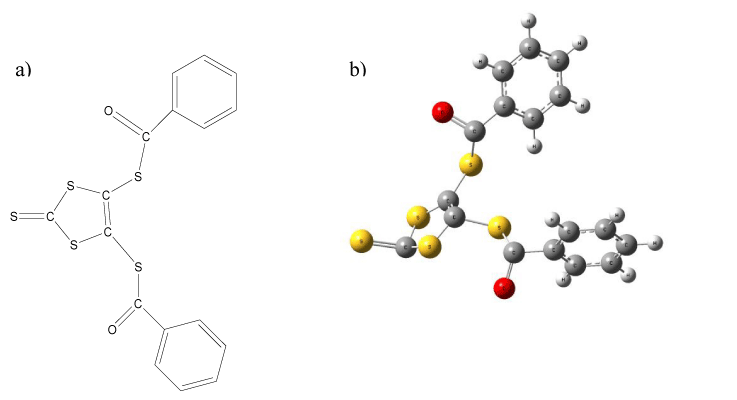
Magnesium an abundant mineral in the body, is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives). Magnesium is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation [1-3]. Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. It contributes to the structural development of bone and is required for the synthesis of DNA, RNA, and the antioxidant glutathione. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm. The principal methods for the trace amounts determination of Mg)III) ions are atomic absorption spectrophotometry(AAS) [4], inductively coupled plasma mass spectrometry (ICP-MS ([5,6], liquid chromatography/inductively coupled plasma atomic emission spectrometry (LC/ICP-AES)[7] , mass spectrometry (MS) [8], isotope dilution mass spectrometry[9], X-ray fluorescence spectrometry[10] , etc. Nearly all of these methods are costly and time consuming. In the meantime, in comparison of PVC membrane electrodes the carbon paste electrodes (CPEs) are one of the subgroup of potentiometric sensors which offer renewable surface, stable response, and low ohmic resistance electrodes. This kind of potentiometric sensors are mostly consisting of the ionophore into a carbon paste matrix including graphite powder dispersed in a non-conductive mineral oil. From the standpoint of mechanical stability, the CPEs can be located between membrane electrodes and all solid state electrodes. According to references and reports the addition of modifier materials such as multiwall carbon nanotubes (MWCNTs) with special physicochemical properties such as ultra-light weights, high mechanical strengths, high electrical conductivities, high thermal conductivities, metallic or semi-metallic behaviors and high surface areas to CPEs, improve the response of this type of sensor [11-14]. Multi-walled carbon nanotubes (MWCNTs) have been recently used in composition of carbon paste electrodes. Using MWCNTs in the carbon paste improves the conductivity and, therefore, conversion of the chemical signal to an electrical signal [15-18]. Literature survey revealed that only one of Mg2+-CPE based on different ionophores have been reported [19]. In this work, based on our previous studies a highly selective and sensitive nano-composite carbon paste composition based on 4,5-Bis (benzoylthio)-1,3-dithiole-2-thione (BZ2dmit) (Figure 1) as the sensing material, nanosilica, multi-walled carbon nanotubes (MWCNTs) and paraffin oil has been developed and tested for the monitoring of Mg (II) ion.
Experimental
Emf measurements: To electromotive force measurement a potentiometric cell was employed including the Mg2+- CPE as the working electrode, an Ag/AgCl electrode (Azar electrode, Iran) as a reference electrode which both electrodes were placed to the glass cell containing Blood Serum and connected to a mili-voltmeter. All electromotive force was carried out with the membrane sensor using the following cell assembly:
Carbon paste electrode | Blood Serum | Ag/AgCl–KCl (satd.)
Materials: Ionophore (BZ2dmit) bought fluka. The graphite powder with a 1–2 μm particle size purchased from Merck. High purity paraffin oil Obtained from Aldrich. The multi-walled carbon nanotubes (MWCNTs) with 10–40 nm diameters, 1–25 μm length and with 95% purity were purchased from Research Institute of the Petroleum Industry (Iran), nitrate and chloride salts of all the used cations (all from Merck Co.) were of the highest available purity and were used without any further purification. Doubly distilled water was used during the experiments.
Carbon paste electrode preparation: For the purpose of preparing the carbon paste electrode the following proceeding was carried out respectively: First, different amounts of the paste ingredients such as ionophore (BZ2dmit), binder (paraffin oil), graphite powder as an inert matrix, NS and MWCNTs as modifier entirely were pooled. Next, the resulting mixture was stirred to get homogenization paste about 30-40 min. Then, the final paste was transferred to the tip of a tube while a copper wire was put into the opposite side of the tube to electrical contact. It should be noted, in order to avoid possible air gaps which mostly lead to boost the electrode resistance, it is necessary to pack up the paste carefully and thoroughly into the tube tip. Last, to replace the new smoothed surface CPE by the old one, the external layer of the carbon paste was polished with soft paper. In the final stage, the electrode was conditioned for 48 h by soaking it in a 1.0×10-3 mol L-1 Mg(NO3)2 solution. Unassayed normal serum samples were analyzed for magnesium. A serum volume of 100 μL was diluted to 5 mL, and potassium was added to give a final concentration of 5000μg/Ml in order to suppress ionization. The sample was acidified with concentrated HCl.
Results and Discussion
Potential response of the electrode
To study the selectivity behavior of designed electrode by potentiometric method, BZ2dmit was employed as a sensing element in preparation of some carbon paste electrode for all of the lanthanide and some of other cations. Magnesium ion due to its appropriate size to the semi cavity of BZ2dmit and the rapid exchange kinetics of the resulting its complexation with ionophore, show the best Nernstian response among all other cations,
Optimization of the MgCPE: One of the main component in ISE (ion selective electrode) which changes the amount of it showing the strong effect on the selectivity of electrode, is known as ionophore or ion carrier (here BZ2dmit).To evaluate the role and determine the best value of BZ2dmit in composite concentration, the diverse amount of BZ2dmit was employed to fabricate a numbers of nano-composite CPEs, according to Table 1 addition of 3% BZ2dmit to CP displays the best response toward the other amounts (No. 2 with the slope of 28.8±0.4 mV decade-1). Ordinarily, the addition of ionophore to the compositions results in increasing the potential responses of the CP electrodes. One of the other ingredients in the composition of the carbon paste which cause to enhance the conductivity of the sensor, and increases the transduction of the chemical signal to electrical signal is MWCNT that adding the proper amount of this material leads to make better the dynamic working range of the sensor. The electrode compositions were modified by adding %1, %2, %3 and %4 wt. of MWCNT to the composition (No. 2 & Nos. 5-7) which led to improvements in the sensitivity of the sensor from the sub-Nerstian value of 34.8±0.7mV decade-1 to 27.1±0.5mV decade-1. On the other hand, it was observed that changing the amounts of the graphite powder as the filler and paraffin, does not significantly change the potential response of the sensor. Finally, the the nanosilica with a high specific surface area has a hydrophobic property that helps to extraction of the ions into the surface of the CPE, so addition of 0.1-0.7% wt. of NS(Nos. 7-9) was found to improve the potential response of Mg (II) activity. In accordance to Table 1, the best response (CPE No. 9) was put out by modified carbon paste electrode based on 0.5% nanosilica, 3% MWCNT, 3% BZ2dmit, 25% paraffin oil and 68.5% graphite powder.
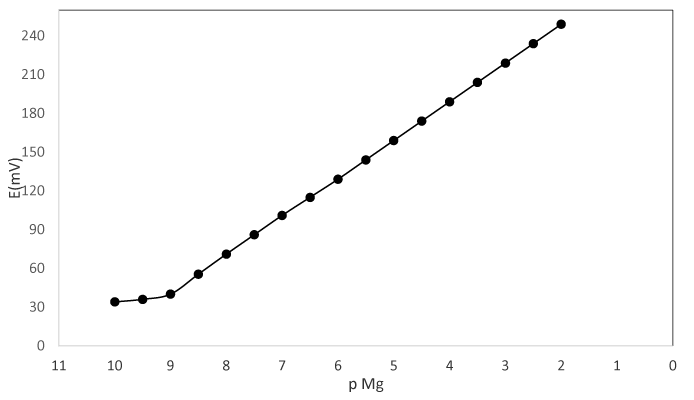
Calibration curve: The potential response of the Mg2+-CP electrode based on BZ2dmit, Fig. 2, which is known in terms of calibration curve displays the widish working linear range from 1.0×10-9 to 1.0×10-2 mol L-1 for optimized BZ2dmit -based Magnesium (II) carbon paste electrode, while according to Nernstian equation the slope of linear part of calibration curve is 29.9±0.7 mV per decade with detection limit of 5.0×10−10 mol L-1 of Magnesium ions concentration which pursuant to the IUPAC recommendations is calculated by crossing of two extrapolated segments of the calibration curve [21-33]. The standard deviation for ten replicate measurements was ±0.6 Mv (Figure 2).
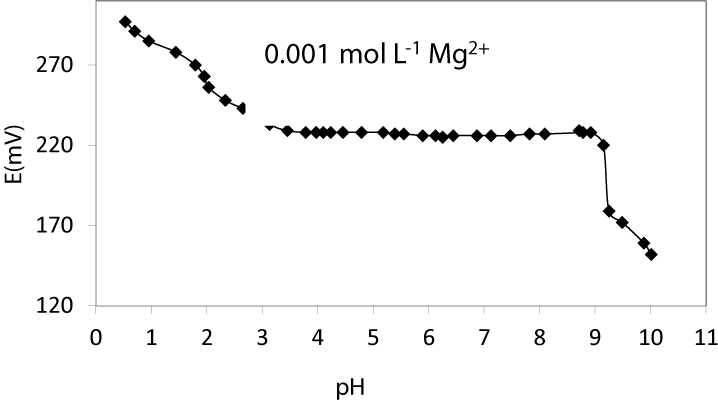
pH effect and response time: In order to study the effect of pH on the performance of the Mg2+-CP sensor, the potentials were determined in the pH range of 1.0-11.0 (the pH was adjusted by using concentrated NaOH or HCl) at one concentrations (1.0×10-3 mol L-1) of Mg2+ and the result is depicted in Figure 3 [34-40]. As it is seen, the potential remained constant from pH 3.4 to 8.9, beyond which some drifts in the potentials were observed. The observed drift at higher pH values could be due to the formation of some hydroxyl complexes of Mg2+ in the solution. At the lower pH values, the potentials increased, indicating that the membrane sensor responded to protonium ions, as a result of the some extent protonation of oxygen atoms of the ionophore.
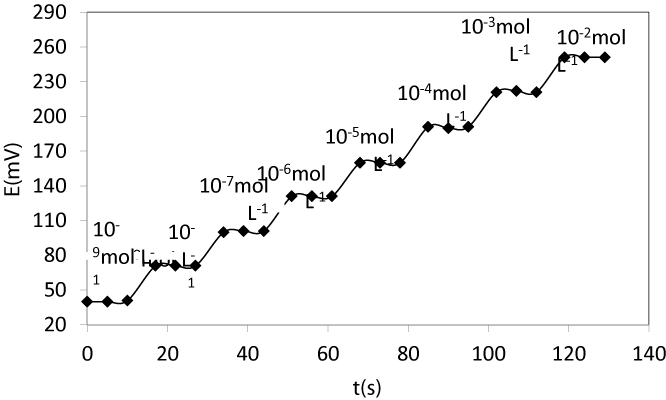
Dynamic response time the MgCPE: Dynamic response time is an important factor for Magnesium carbon paste electrodes [41-45]. In this study, the practical response time was recorded by changing solution with different Mg+2 concentrations from 1.0×10-9 to 1.0×10-2 mol L-1. The actual potential versus time trace for the electrode based on BZ2dmit is shown in Figure 4. As can be seen, the electrode reaches the equilibrium response in a very short time of about 7 s.
Selectivity studies of the MgCPE: The priority of primary ion over interfering ions (alkaline, alkaline earth, lanthanides, transition and heavy metals) is known as the selectivity coefficients which indicate the disturbance of other ions on the response of designed electrode [103-108]. This main factor of ISE was measured graphically at this work by the match potential method (MPM). to determine the selectivity coefficients by matched potential method, first, the potential of the specific activity of Mg+2 ions solution while adding to a reference solution was measured, next, the apparent amount of interfering ions solution was sequentially added to the same reference solution in the other experiment to obtain the potential response which matches the one acquired previously by adding Mg+2 ion. In the last stage, the final results getting by the following equation that is the ratio of primary ion (A) activity changes to the interfering ion (B).
The resulting selectivity coefficients, which are listed in Table 2, demonstrated the negligible disturbance of various cations for the proposed electrode which bodes a good performance of the designed CPE toward the Mg2+ions and specific interaction between Mg+2 ions and BZ2dmit.
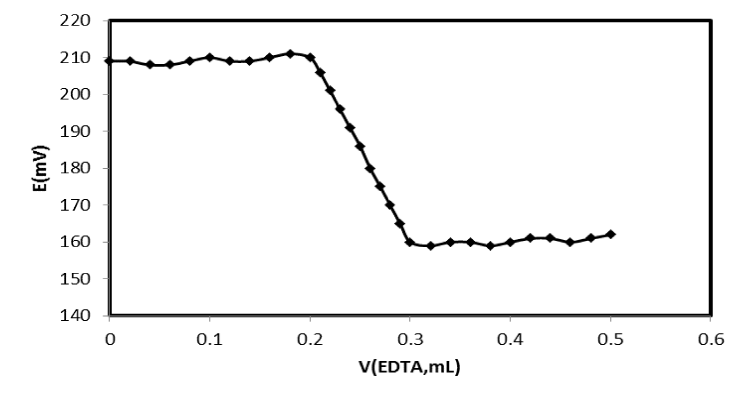
Analytical application: The proposed Mg2+-CPE was successfully used as an indicator electrode in the titration of Unassayed normal serum samples were analyzed for magnesium. A serum volume of 100 μL was diluted to 5 mL, and potassium was added to give a final concentration of 5000μg/mL in order to suppress ionization. The sample was acidified with concentrated HCl with a 1.0×10−2 mol L-1 EDTA. The resulting titration curve is given in Figure 5, demonstrating that the amount of Mg(II) ion in the solution can be determined with the electrode.
Lifetime of the Sensor: Life time is one of the imprtant factor for any sensor. Literature survey revealed that for most ion selective sensors, lifetimes range are between 4-10 [46-48]. After this time, a significant change will observed in the slope and detection limit of the sensor. The lifetime of the proposed nano-composite base Mg(II) sensor was evaluated for a period of 18 weeks, during which the sensor was used for two hours per day. The changes in the slope and detection limit of the modified CPE were measured and the results are given in Table 3. As can be seen from Table 3, the proposed nano-composite Mg(II) sensor can be used for at least 17 weeks. Without significant changes on its slope and detection limits. After 17 weeks, this time, a significant decreasing in the slope from 29.9±0.7 to 28.1±0.1 mV decade-1 and a gradual increasing in detection limit from 5.0×10-10 to 2.5×10-9 was observed. This phenomenon is most probaly due to the loss of room temperature ionic liquid (RTIL) and BZ2dmit from the composition of the fabricated nano composite modified Mg(II) carbon paste electrode.
Conclusions
In this work, 4,5-Bis (benzoylthio)-1,3-dithiole-2-thione (BZ2dmit) with heteroatoms including oxygen and sulfur was employed as an active material to play the role of selector in construction of Mg(II) nanocomposite carbon paste electrode which modified by MWCNT and nanosilica. The modified Mg+2-CPE in comparison of recently designed PVC membrane electrode based on same ionophore to determination of Mg (II) ion exhibited the better potentiometric response in terms of sensitivity, Nernstian slope, linear range, and response stability. The created Mg+2-CPE showed a Nernstian response (29.9±0.7 mV decade-1) in the range of 5.0×10-9-1.0×10-2 mol L-1 with detection limit of 5.0×10-10 mol L-1. The working pH range which the response of the sensor is independent from H3O+ ion concentration changes is about 3.4-8.9. The nano-composite based Mg(II) sensor displayed good selectivity, response time (about 7 s). Further, the developed electrode can be employed successfully to analyze the applications such as potentiometric titration Determination of Magnesium in Blood Serum and monitoring in various mixtures of interfering ions.
The authors acknowledge the financial support kindly offered by the Research Council of Mashhad Branch Islamic Azad University.
- Institute of Medicine (IOM) (1997) Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press .
- Rude RK (2010) Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare 527-537 .
- Rude RK (2012) Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins 159-175 .
- Van Loon JC, Galbraith JH, Aarden HM (1971) The determination of yttrium, europium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium in minerals by atomic-absorption spectrophotometry Analyst 96: 47-50 .
- Barbaro M, Passariello B, Quaresima S, Casciello A, Marabini A (1995) Analysis of Rare Earth Elements in Rock Samples by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Microchemical journal 51: 312-318 .
- Stetzenbach KJ, Amano M, Kreamer DK, Hodge VF (1994) Testing the Limits of ICP-MS: Determination of Trace Elements in Ground Water at the Part-Per-Trillion Level Groundwater 32: 976-985 .
- Yoshida K, Haraguchi H (1984) Determination of rare earth elements by liquid chromatography/inductively coupled plasma atomic emission Analytical Chemistry 56: 2580-2585 .
- Walder AJ, Platzner I, Freedman PA (1993) Isotope ratio measurement of lead, neodymium and neodymium–samarium mixtures, Hafnium and Hafnium–Lutetium mixtures with a double focusing multiple collector inductively coupled plasma mass spectrometer. J Anal At Spectrom 8: 19-23 .
- Yuan HL, Gao S, Zong CL, Dai MN (2009) Sectional power-law correction for the accurate determination of lutetium by isotope dilution multiple collector-inductively coupled plasma-mass spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy 64: 1228-1234 .
- Eby GN (1972) Determination of rare-earth, yttrium, and scandium abundances in rocks and minerals by an ion exchange-x-ray fluorescence procedure Anal Chem 44: 2137-2143 .
- Ajayan PM (1999) Nanotubes from Carbon. Chem Rev 99: 1787-1800 .
- Goyal RN, Oyama M, Gupta VK, Singh SP, Sharma RA (2008) Sensors for 5-hydroxytryptamine and 5-hydroxyindole acetic acid based on nanomaterial modified electrodes Sens. Actuators B 134: 816-821 .
- Liu L, Wang L, Yin H, Li Y, He X (2006) The Preparation and Application of Bismuth (III) Ion‐Selective Electrode Based on Nanoparticles of Bismuth Sulfide. Analytical Letters 39: 879-890.
- G.Li, H. Xu, W.J. Huang, Y. Wang, Y.S. Wu, and R. Parajuli (2008) Mea Sci Technol 19: 065203.
- Ganjali MR, Khoshsafar H, Faridbod F, Shirzadmehr A, Javanbakht M, et al. (2009) Room Temperature Ionic Liquids (RTILs) and Multiwalled Carbon Nanotubes (MWCNTs) as Modifiers for Improvement of Carbon Paste Ion Selective Electrode Response; A Comparison Study with PVC Membrane. Electroanalysis 21: 2175-2178.
- Maleki N, Safavi A, Tajabadi F(2006) High-Performance Carbon Composite Electrode Based on an Ionic Liquid as a Binder. Anal Chem 78: 3820-3826 .
- Rezaei B, Damiri S (2008) Multiwalled Carbon Nanotubes Modified Electrode as a Sensor for Adsorptive Stripping Voltammetric Determination of Hydrochlorothiazide. IEEE Sensors 8: 1523 .
- Siswana M, Ozoemena KI, Nyokong T (2008) Electrocatalytic Detection of Amitrole on the Multi-Walled Carbon Nanotube – Iron (II) tetra-aminophthalocyanine Platform. Sensors 8: 5096-5105 .
- Norouzi P, Pirali-Hamedani M, Ranaei-Siadat SO, Ganjali MR (2011) Nanocomposite Carbon Paste Sensor for Selective
- Determination of Lu(III) Ion. Int. J Electrochem Sci 6: 3704-3713 .
- De Marcol R, Clarke G, Pejcic B (2007) Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis 19: 1987-2001 .
- Kim JS, Ohki A, Ueki R, Ishizuka T, Shimotashiro T, et al. (1999) Cesium-ion selective electrodes based on calix[4]arene dibenzocrown ethers. Talanta 48: 705-710 .
- Ganjali MR, Norouzi P, Faridbod F, Riahi S, Ravanshad J, et al. (2007) Determination of Vanadyl Ions by a New PVC Membrane Sensor Based on N, N'-bis-(Salicylidene)-2,2-Dimethylpropane-1,3-Diamine Ieee Sensors J 7: 544-550 .
- Zamani HA, Zabihi MS, Rohani M, Zangeneh-Asadabadi A, Ganjali MR, et al. (2011) Quantitative monitoring of terbium ion by a Tb3+ selective electrode based on a new Schiff's base. Mater Sci Eng C 31: 409-413 .
- Harwood JE (1969) The use of an ion-selective electrode for routine fluoride analyses on water samples. Water Research 3: 273-280 .
- Zamani HA, Ganjali MR, Behmadi H, Behnajady MA (2009) Fabrication of an iron(III) PVC-membrane sensor based on bis-benzilthiocarbohydrazide as a selective sensing material. Mater Sci Eng C 29: 1535-1539 .
- Mahajan RK, Kaur I, Lobana TS (2003) Talanta. 59: 101.
- Ganjali MR, Norouzi P, Mirnaghi FS, Riahi S, Faridbod F (2007) Lanthanide Recognition: Monitoring of Praseodymium(III) by a Novel Praseodymium(III) Microsensor Based on N′-(Pyridin-2-Ylmethylene) Benzohydrazide. Ieee Sensors J 7: 1138-1144 .
- Zamani HA, Rajabzadeh G, Masrornia M, Dejbord A, Ganjali MR (2009) Determination of Cr3+ ions in biological and environmental samples by a chromium(III) membrane sensor based on 5-amino-1-phenyl-1H-pyrazole-4-carboxamide. Desalination 249 (2009) 560-565 .
- Craggs A, Moody GJ, Thomas JDR (1974) PVC matrix membrane ion-selective electrodes. Construction and laboratory experiments J Chem Educ 51: 541 .
- Coetzee JF, Istone WK (1980) Copper ion-selective electrode for evaluation of free energies of transfer of copper(II) ion from water to other solvents. Anal Chem 52: 53-59 .
- Zamani HA, Ganjali MR, Norouzi P, Meghdadi S (2008) Application of Novel Praseodymium (III) PVC‐Membrane Electrode for Determination of Pr(III) Ions in Soil and Sediment Samples. Anal Lett 41: 902-916 .
- Altura BT, Altura BM (1991) Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem 10: 90-98 .
- Naddaf E, Zamani HA (2009) Samarium(III)-PVC-Membrane Sensor Based on Ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline Anal Lett 42: 2838-2852 .
- Zamani HA, Abedini-Torghabeh J, Ganjali MR (2006) A Highly Selective and Sensitive Calcium(II)-Selective PVC Membrane Based on Dimethyl 1-(4-Nitrobenzoyl)-8-oxo-2,8-dihydro-1H-pyrazolo[5,1-a]isoindole-2,3-dicarboxylate as a Novel Ionophore. Bull Korean Chem Soc 27: 835-840 .
- Zamani HA, Zanganeh-Asadabadi A, Rohani M, Zabihi MS, Fadaee J, et al. (1999) A Ho(III) potentiometric polymeric membrane sensor based on a new four dentate neutral ion carrier. Mater Sci Eng C 33: 984-988 .
- Zamani HA (2011) Chem 8: S97.
- Zamani HA, Ganjali MR, Norouzi P, Adib M (2007) Cobalt(II) Ion Detection in Electroplating Wastewater by a New Cobalt Ion-Selective Electrode Based on N′-[1-(2-thienyl)ethylidene]-2-furohydrazide. Sensor Lett 5: 522-527 .
- Zamani HA, Ganjali MR, Adib M (2007) Fabrication of a new samarium(III) ion-selective electrode based on 3-{[2-oxo-1(2h)-acenaphthylenyliden]amino}-2-thioxo -1,3-thiazolidin-4-one J Braz Chem Soc 18: 215 .
- Zamani HA, Ganjali MR, Norouzi P, Tajarodi A, Hanifehpour Y (2007) Fabrication of a Cobalt(Ii) Pvc-Membrane Sensor Based on N-(Antipyridynil)-N'-(2-Methoxyphenyl)Thiourea. J Chil Chem Soc 52: 1332-1337 .
- Zamani HA, Rajabzadeh G, Firouz A, Ganjali MR (2007) Determination of copper(II) in wastewater by electroplating samples using a PVC-membrane copper(II)-selective electrode. J Anal Chem 62: 1080-1087 .
- Zamani HA, Mohammadhossieni M, Nekoei M, Ganjali MR (2010) Determination of Erbium Ions in Water Samples by a PVC Membrane Erbium-Ion Selective Electrode. Sensor Lett 8: 303-307 .
- Abedi MR, Zamani HA (2008) Barium(II)-PVC Membrane Sensor Based on 4-4′-Methylenediantipyrine as a Neutral Carrier. Anal Lett 41: 2251-2266 .
- Zamani HA, Rohani M, Mohammadhosseini M, Ganjali MR, Faridbod F, et al. (2011) Quantitative Monitoring of Erbium Ion in Alloy Samples by a Erbium Selective Sensor. Sensor Lett 9: 1745-1749 .
- Zamani HA (2011) N1,N2-Bis[1-(2-hydroxyphenyl)methylidene]ethanedihydrazide as a neutral ionophore for preparation of a new Ho3+ PVC-membrane sensor. Chinese Chem Lett. 22: 701-704 .
- Ganjali MR, Motakef-Kazemi N, Norouzi P, Khoee S (2009) A Modified Ho3+ Carbon Paste Electrode Based on Multi-walled Carbon Nanotubes (MWCNTs) and Nanosilica. Int J Electrochem Sci 4: 906-913 .
- Ganjali MR, Khoshsafar H, Faridbod F, Shirzadmehr A, Javanbakht M, et al. (2009) Room Temperature Ionic Liquids (RTILs) and Multiwalled Carbon Nanotubes (MWCNTs) as Modifiers for Improvement of Carbon Paste Ion Selective Electrode Response; A Comparison Study with PVC Membrane. Electroanalysis 21: 2175-2178.
- Ganjali MR, Motakef-Kazemi N, Faridbod F, Khoee S, Norouzi P (2010) Determination of Pb2+ ions by a modified carbon paste electrode based on multi-walled carbon nanotubes (MWCNTs) and nanosilica. J Hazard Mater 173: 415-419 .

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
