Open Journal of Analytical and Bioanalytical Chemistry
Analysis are of the hidden properties of the macromolecular system as an example of the reaction centers of bacteria Rhodobacter sphaeroides
YM Barabash1*, TV Serdenko1,PP Knox2 and OYu Bondarenko3
2MV Lomonosov, Moscow State University, 119991 Moscow, Russia
3Institute of Plant Physiology and Genetics, NAS Ukraine, Vasylkivska St. 31/17, Kyiv, 03022, Ukraine
Cite this as
Barabash YM, Serdenko TV, Knox PP, Bondarenko O (2019) Analysis are of the hidden properties of the macromolecular system as an example of the reaction centers of bacteria Rhodobacter sphaeroides. Open J Anal Bioanal Chem 3(1): 057-064. DOI: 10.17352/ojabc.000012Relevant: Is the study of the response of biological macromolecules to external stimuli. Often the reaction of macromolecules has an effect of structural self-regulation. In this case, their reaction is not only external influence, but also the spatial-temporal motions of the macromolecule. In this situation deserves the attention of electronic-conformational interactions of macromolecules. As objects are isolated Reaction Centers (RC) of bacteria Rhodobacter sphaeroides, the structure of which is well studied. During prolonged illumination, the RC occurs intramolecular electron transfer of RC, the kinetics of which has expressed as a sum of three different exponential functions with negative values decrements. In this case, can considered two models of electron transfer with variable or constant with time of the kinetic parameters.
Problem: Arises of analyzing the kinetics of electron transfer, which influenced by the latent parameters of the RC protein structure, which are difficult to determine in the experiment.
Aim: Of the work is to determine the features of intramolecular electron transfer between the conformational states of RC, which are associated with a change in the structure of the macromolecule.
Result: It was determined that the position of the maxima of the wavelet transform spectrum of the logarithmic derivative of the electron transfer kinetics corresponded to the minimax values of the time dependence of the probability density of the presence of an electron in various redox-conformational states of the RC (population of redox states). Minimax values of the population of RC states corresponded to: 1–6s, 30–60s, 100–140s for various parameters of RC photoexcitation.
Conclusion: That the existence of minimax values of the probability density is the electron in the conformational states of the RC can related to the effects of structural self-regulation of the electron transfer process.
Abbreviations
RC: Reaction Center; P(BChl): is an Electron Donor Represented by a Bacteriochlorophyll Dimer; B: Bacteriochlorophyll; H: Bacteriopheophytin; BA: First Acceptor (Bacteriochlorophyll); HA-Primary Electron Acceptor (Bacteriopheophytin); Fe: Non-Heme Iron; QA,QB: Electron Acceptors (Quinones); LDAO: Lauryldimethylamine Oxide (Detergent); Xi: The time Dependence of the Probability Density of Tinding an Electron (population) in the Conformational States of the Reaction Center; Texp: Time the Light Exposure of Reaction Center; Ai: The Weight of the Exponential Component of the main Reaction; di: Is the Exponential Component (decrement) of the main Reaction; ki-j: The Rate Constants of the Kinetic Reaction in the Balance Equations
Introduction
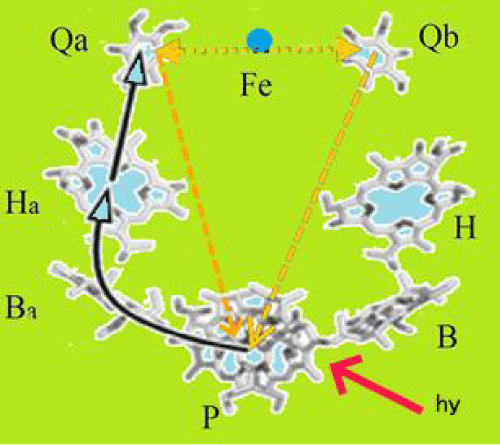
Membrane protein-pigment complexes isolated, photosynthetic reaction centers of Rhodobacter sphaeroides are macromolecular systems that used to study the physical mechanisms of electron and proton transfer in biological structures and the regulatory role of molecular dynamics. The structure of the RC has been well studied. When the photo is excited by the RC, the absorbed light quantum causes the excitation of a bacteriochlorophyll dimer (RC donor). An excited electron passes through the bacteriochlorophyll monomer to pheophytin, then from pheophytin to acceptors (Quinones QA,QB), overcoming a distance of 40Å. On each of these cofactors (Figure 1) of the electron transfer chain, the structural and dynamic properties of RCs play an important role [1-4].
They play an equally important role in the RC relaxation processes, when the electron returns to the bacteriochlorophyll dimer after switching off the light. Upon a prolonged photoexcitation of RC the process of electron transfer repeats and becomes cyclic. Once again the absorption of a quantum of light and the transfer of an electron from a donor to an acceptor QB takes place. As a rule, the kinetics of cyclic electron transfer has a multi-exponential character with negative decrements both at the stage of oxidation of the donor and at the stage of its reduction. The kinetics of cyclic electron transfer was associated with the probability density (population of state) of the electron being in various electron-conformational states RC, that is, with the probability of finding the RC in different electron-conformational states. At the same time, the mathematical form of the exponential functions has no clear features. Their parameters are interrelated and depend on the parameters of the photo excitation RC [5-8]. This makes difficult to physically interpret the exponential components of the electron transfer kinetics. Therefore, further analysis of the kinetics of oxidation and reduction of the donor RC it carried out using two RC models that used the multi exponential nature of the electron transfer kinetics. In one model, a two-level scheme with time-varying parameters was considered. The kinetics of cyclic electron transfer was analyzed using wavelet analysis. Wavelet analysis consists in convolving a wavelet with the function under study and allows revealing its frequency-time characteristics. However, the features of the studied function are difficult to interpret, since they depend on the type and parameters of the used wavelets [9,10]. The second model represented by a four-level scheme with parameters constant in time. It used a system of four differential equations with constant coefficients and one equation of material balance. The identification of the system of equations has carried out by comparing one of the solutions of differential equations with the experimental kinetics of electron transfer [11]. This made it possible to determine the time dependence of the probability density of finding RC in four electron-conformational states for different photo excitation conditions. However, determining the values of the constant coefficients of a system of differential equations is not a simple task.
The purpose of work is to analyze the features of the kinetics of electron transfer of RC in various models of electron transfer and to estimate the assumption that these features are due to the regulatory role of the RC protein.
Materials and Methods
Isolated wild type RC Rhodobacter sphaeroides were provided by the Department of Biophysics MSU Moscow State University M.V. Lomonosov. They has placed in a 0.01M Na-P buffer with pH of 7.2, which contained 0.05% LDAO. RCs were studied using two-channel PC-controlled spectrometer. In the absorption measurement channel, light was used with a frequency of 5kHz and intensity (0.2µW/cm2, λ=865±10nm), which provided absorption measurements in the range of values of 0÷1 with an accuracy of 0,0005. The RC excitation channel used light with a wavelength of λ=870±50nm and an intensity of 0÷10mW/cm2 with a step of 5µW/cm2. The time measurement step did >=0.01 seconds. The measuring cuvette had dimensions of 3×1×2.5cm with a wall thickness of 2mm. After dilution, the RC suspension with a concentration of 10-6M was kept in the dark at room temperature for 12 hours (the dark-adapted RC state). The light-adapted state was formed by illuminating the RC by light pulses of different durations (Texp=1, 5, 50, 120, 300, 600s) with an intensity of 0.5, 1.5, 3.5mW/cm2. After the end of illumination and the attainment of dark absorption value, the RC solution was additionally kept in the dark for 1500s to reach an equilibrium state [12]. Then the lighting of RC was repeated with the next light pulse. The relative number of absorption centers and the rate of RC transition from one state to another were determined from the kinetics of fading of the 865nm line of the RC absorption spectrum, and were associated with the kinetics of electron transfer from donor to acceptor [6]. The change in RC absorption determined the time dependence of the probability of the electron being on the donor (population of the RC donor). The probability has normalized to the maximum change in the absorption of the RC suspension at a wavelength of 870nm with an exciting light intensity of 7.2mW/cm2. For the analysis of electron-conformational processes in RC two models of electron transfer were used: a two-level model [6,7] and a model with four sub states [12]. According to the two-level model, the RCs are in the ground (1) state when the electron localized on the donor P. When the light quantum is absorbed, the electron moves to the QB acceptor, and the RC goes to the excited state. The kinetics of electron transfer in this model is determined by the rate constant of direct (k01(t)) electron transfer at the RC illumination stage (first stage of electron transfer) and the electron back transfer rate constant (kT10(t)) at RC relaxation stage after illumination (second stage of transfer electron). The kinetics of the first stage of photo-induced electron transfer RC can be described by a differential equation and a material balance equation:
dp(t)/dt=-k01(I)·p(t)+k10(I,t)·(1-p(t)), p(t)+q(t)=1 (1)
where p(t), q(t) is the probability density of finding the RC in such a state when the electron is on the donor (acceptor), i.e. their populations, with the initial condition p(0)=1 q(0)=0.
The kinetics of electron transfer from the acceptor to the donor (upon restoration of the donor) after switching off the light (I0=0) is:
dq(t)/dt = -kT10(t)·q(t) or dq(t)/dt·q(t) = -kT10(t) (2)
where kT10(t) is the rate constant of electron transfer from acceptor (Q) to donor (P) after switching off the light.
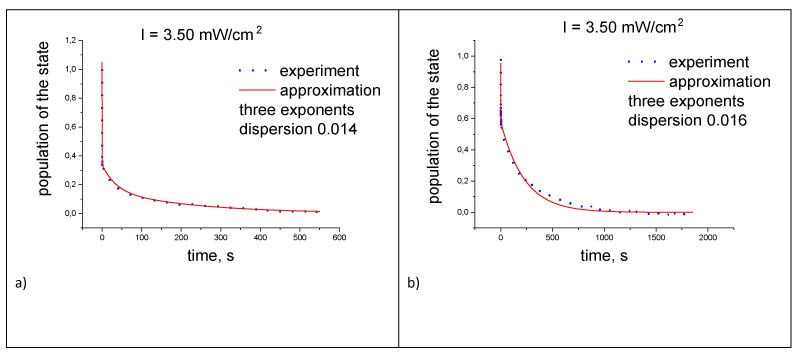
The second stage of electron transfer is simpler than the first stage. The left side of expression (2) (dq(t)/dt·q(t)) is the logarithmic derivative of the probability of finding an electron on the acceptor q(t). It determines the time dependence of the electron transfer rate constant from the acceptor to the donor after turning off the light. The kinetics of recovery of the RC donor during the relaxation of the RC was approximated by the sum of three exponential functions, , i = 1, 2, 3), which had the minimum error [13] (Figure 4a). Then, the dependence of the logarithmic derivative on time as the sum of three exponential functions was analyzed using the continuous wavelet transform (CWT). The kinetics of the first stage of electron transfer during photo excitation of the RC was also presented as the sum of three exponential functions (Table 1). In this case, the logarithmic derivative of the probability p(t) of finding an electron on the donor under RC illumination is expressed not only in terms of electron transfer rate constants. However, if we do not consider the equilibrium absorption values, the absorption kinetics when illuminating the RC could also be well approximated by the sum of three exponential functions (Figure 4b). This allowed us to study the kinetics of electron transfer using the logarithmic derivative p(t). CWT determines the characteristics of the signal, their frequency and temporal localization. The CWT spectrum represented by the formula:
where s(t) is the function under analysis, ψ(t) is the wavelet, a is the parameter that determines the width of the wavelet, and b is the wavelet time shift parameter.
A complex Morlet wavelet used, which is a harmonic function with a Gaussian envelope:
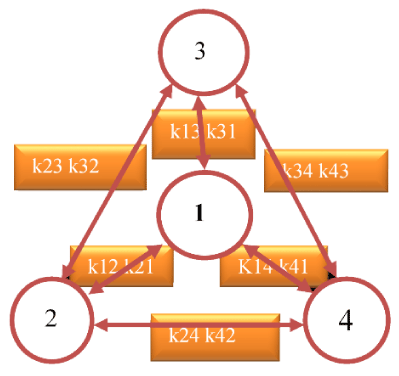
This wavelet has good spectrogram convergence. This minimizes the time-frequency uncertainty and ambiguity of interpretation of the obtained wavelet spectra [14]. However, the parameters of the characteristics of the spectrum depend on the type of wavelet used [14,15]. It has known that the system of N-homogeneous differential equations with constant coefficients and with the material balance equation has a solution in the form of the sum N-1 of exponential functions [12]. The parameters of the electron transfer kinetics it presented as a sum of three different exponential functions that have a minimum mean-square approximation error (Tables 2-4). Therefore, a system of four differential equations with constant coefficients used to study the kinetics of electron transfer. The identification of a system of equations is a multi-parameter optimization task. Identification has performed using a software product that found the values of the rate constants of the kinetic equations. The values of the constants corresponded to the minimum value of the discrepancy between the parameters of the exponential functions, which are one of the solutions of the differential equations and the result of the approximation of the kinetics of cyclic electron transfer. Two systems of differential equations used. One system describes the first stage of electron transfer when the RC is illuminated (donor oxidation). Another system describes the second stage of electron transfer after illumination (donor recovery) RC [12]. The kinetic scheme for finding RCs in various electron-conformational states shown in Figure 2. The 1-RC state corresponds to the probability density of finding an electron on the RC donor. The 2-RC, 3-RC and 4-RC states correspond to the probability density of finding the electron on the RC acceptor. The determination of the coefficients and the solution of differential equations gives the kinetics of the probability of finding RCs in various electron-conformational states. The data on the features of the kinetics of the states of the RC compared with the data of the wavelet analysis.
Experimental Results and Discussion
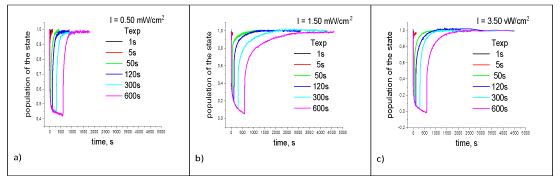
The measurement protocol included the setting of the intensity, the duration of the RC excitation pulse, and the time step of the measurements. After the start of measurements, the value of the dark absorption of the suspension has recorded during the first 20 steps. When the light pulse was turned on and Texp<5 seconds, the data were recorded in 0.01s increments, if Texp>5 seconds, the data were recorded in increments (0.1s). After turning off the light, the data were recorded for the first 5 seconds with a step of 0.01s, then with a step of 0.1s until the dark absorption value of the suspension RC was reached, and then with a step of 1s for 1500s. This time mode of absorption measurements of the RC provided the necessary accuracy of the approximation of the absorption by exponential functions. In Figure 3a,b shows the electron transfer kinetics of the RC for various parameters of photo excitation.
First, a measurement has made in Figure 3a, then Figure 3b and Figure3c, according to the measurement protocol described above.
In Figure 4 shows the approximation by three exponential functions of the time dependence of the probability density of finding an electron (population kinetics) on the donor (acceptor) of RC during photo excitation of RC. This number of functions corresponded to the minimum mean square error [15]. These data show that the kinetics of the donor population when illuminated by RC can also has approximated by three exponential functions.
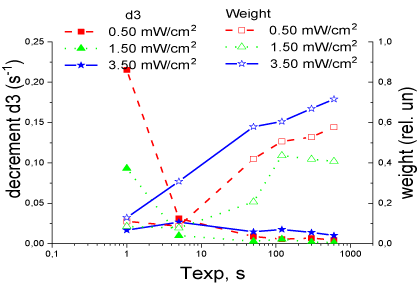
The calculated parameters of exponential functions (decrement and weight) for various photo modes of RC activation (Tables 1–4) used in wavelet analysis and in identifying the system of differential equations of electron transfer in RC.
The data in tables 2-4 showed (Figure 5) that the parameters of the slowest exponential component of the absorption kinetics during relaxation of the RC after turning off the light can be associated with structural changes in the RC protein that occurred during photo excitation of the RC. The remaining exponential components of the electron transfer kinetics require additional analysis.
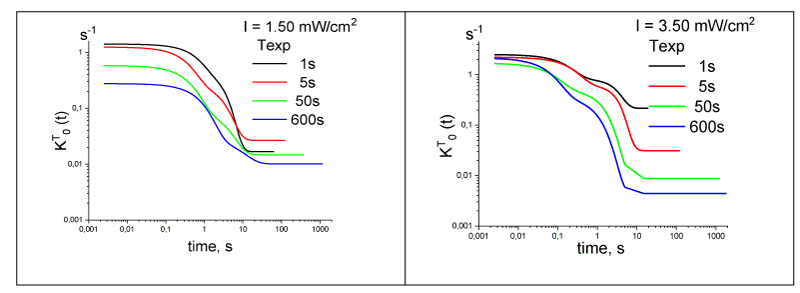
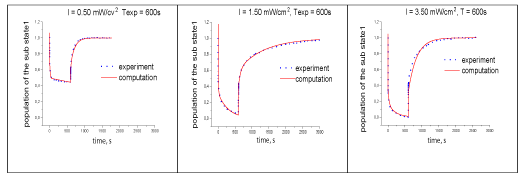
The time dependence of the rate constant kT10(t) of the reverse electron transfer from the acceptor to the donor during RC relaxation is shown in Figure 6. It is S-shaped with three characteristic sections. The values of the constant at the beginning and end of the relaxation differ by an order of magnitude. This suggests that the two-level model with constant electron transfer parameters inadequately describes the experimental data, despite the frequent use of modified solutions of this model. As mentioned, the parameters of the exponential components of the kinetics of electron transfer used as the objective function in the problem of determining the rate constants for two systems of differential equations. One described the electron transfer kinetics when the reaction center was illuminated. Another system described the electron transfer kinetics after turning off the light. The solution of differential equations determines the density kinetics of the probability of finding an electron (population) of the four states of the reaction center. Comparison of the calculated population of the donor (state 1) of the reaction center with the experimental kinetics of electron transfer (Figure 7) shows their satisfactory agreement.
This is observed at different modes of photo excitation of the reaction center, which allows us to consider the reaction center as a system of 4 electron-conformational states when studying the electron transfer process. Tables 5-7 show the calculated rate constants for the differential equations of electron transfer for exciting light of RC of different intensity with a duration of 600s of the kinetic coefficients for an exciting light of duration 600s.
In first line of tables, denote constants of rate of electron transfer of RC.
In second line shows values of constants rate of electron transfer under illumination RC.
Third line shows the values of electron transfer rate constants after the termination of the illumination of RC.
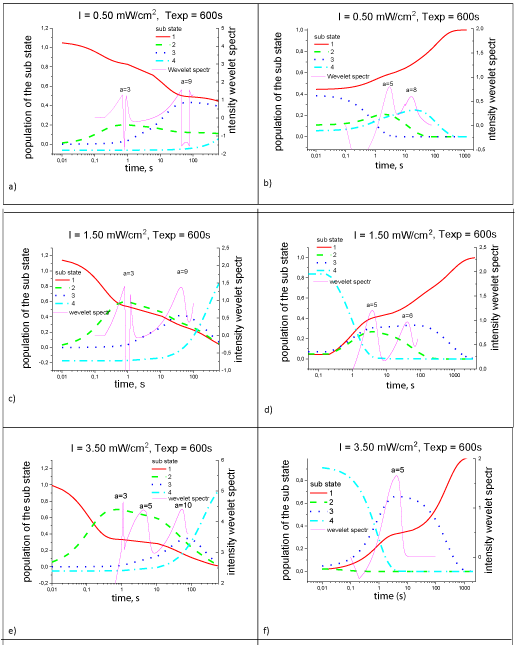
In Figure 8 shows the wavelet spectra of the logarithmic derivative of the experimental kinetics of electron photo transfer and the calculated populations of the four states of the reaction center. These curves have pronounced features both at the stage of photo excitation of the reaction center (oxidation of the donor) and at the stage of relaxation of the reaction center (restoration of the donor) after turning off the light. At the stage of photo excitation of the reaction center, the first narrow peak of the wavelet spectrum (a=3) corresponds to a maximum (1f) of the population of the second state. The second, broader peak (a=9) of the spectrum corresponds to a maximum (60s) of the population of the third state (Figure 7a,c,e). At the stage of relaxation of the reaction center (Figure 7b,d,f), two peaks of the wavelet spectrum (a=5,6) correspond to maxima (3, 60s) of populations of 2, 3 states and minimum of the 4th state of the reaction center. The times are hierarchical. The maxima of the probabilistic dependences of the presence of RC in various electron-conformational states are due to the influence of a change in the structure of the RC during cyclic electron transfer. This is especially true for a simpler RC relaxation process after the photo excitation stops Figure 8 (b,d,f). The minimax values of the probability of realization of electron-conformational states of the RC occur when a long-lived pair of localized charges formed during cyclic electron transfer. In this case, there is a change in the electron density in the structures of the protein, which are located near the electron transfer path. As a result, there occurs a change in the free energy ΔGо, the overlap of the wave functions of the electron carriers and the reorganization energy (λ) protein, which is difficult to measure [16,17]. The energy λ of the protein reorganization corresponds tunneling of the electron without activation as result of a change in the nuclear coordinates of the nearest environment of the electron transfer path. A mutual relationship arises between the features of the kinetics of electron transfer and the protein motions of the reaction center, which have a fading character. The effect of structural self-regulation of electron transfer appears.
Conclusions
The kinetics of photo stimulated electron transfer of the RC can represented by the sum of three different exponential functions with negative exponents.
The reaction center model in the form of a system of 4 redox-conformational states corresponds to two systems of four differential equations with constant coefficients separately for the photo-excitation stage of the RC and the stage relaxation of the RC after the light is turned off.
Temporal dependences, the probability density of the RC in four redox-conformational states and the wavelet spectrum of the logarithmic derivative of the electron transfer kinetics have extrema, the time parameters of which coincide with each other in different modes of RC photoexcitation.
The extrema of the kinetics of the probability density of the redox conformational states of the reaction center and the wavelet spectrum lie in the range of 1, 3, 60sec. They have a hierarchical character and has caused by spatio-temporal movements of the RC protein as result of the manifestation of the effect of self-regulation of the electron transfer kinetics.
- Feher G, Allen JP, Okamura MY, Rees DC (1989) Structure and function of bacterial photosynthetic reaction centres. Nature 339: 111-116. Link: https://go.nature.com/2lXwxeY
- Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3Å resolution. Nature 318: 618-624. Link: http://bit.ly/2kpIb1I
- Andréasson U, Andréasson LE (2003) Characterization of a semi-stable, charge-separated state in reaction centers from Rhodobacter sphaeroides. Photosynth Res 75: 223-233. Link: http://bit.ly/2m03t6f
- Potter JA, Fyfe PK, Frolov D, Wakeham MC, Van Grondelle R, et al. (2005) Strong effects of an individual water molecule on the rate of primary charge separation in the Rhodobacter sphaeroides reaction centre. J Biol Chem 280: 27155-27164. Link: http://bit.ly/2lSs652
- Xu Q, Gunner MR (2003) Trapping Conformational Intermediate States in the Reaction Center Protein from Photosynthetic Bacteria. Biochem 40: 3232-3324. Link: http://bit.ly/2kQfGu3
- Rubin AB (2017) Compendium of Biophysics. Wiley 660. Link: http://bit.ly/2kNwaDe
- Deshmukh SS, Williams JC, Allen JP, Kálmán L (2011) Light-induced conformational changes in photosynthetic reaction centers: dielectric relaxation in the vicinity of the dimer. Biochem 50: 340-348. Link: http://bit.ly/2kE1Vik
- Kharkyanen VN, Barabash YM, Berezetskaya NM, Lukashev EP, Knox PP, et al. (2011) Peculiarities of light-induced slow protein dynamic in the photosynthetic reaction center. Chem Phys Lett 512: 113-117. Link: http://bit.ly/2kQgb7p
- Golbeck J, Est VD (2014) The Biophysics of Photosynthesis. Springer 465. Link: http://bit.ly/2miGnYN
- D'jakonov VP (2000) Wavelets, from theory to practice. Moscow: SOLON-Press. Russians.
- Barabash YM, Serdenko TV, Knox PP, Golub AA (2019) Use of a system of differential equations to analyze the functioning of a catalytic bio macromolecule under non-equilibrium conditions. Heliyon 5: e02108. Link: http://bit.ly/2kQdGSB
- Serdenko TV, Barabash YM, Knox PP, Seifullina NKH (2016) The kinetic model for slow photoinduced electron transport in the reaction centers of purple bacteria. Nanoscale Res Lett 11: 286. Link: http://bit.ly/2kE8lho
- Barabash YM, Lyamets AK (2016) A method of decomposition of the basic reaction of biological macromolecules into exponential components. Nanoscale Res Lett 11: 544. Link: http://bit.ly/2kox9ty
- Maks Zh (1983) Methods and techniques for processing signals in physical measurements. Moscow.
- Stark HG (2005) Wavelets and Signal Processing. Link: http://bit.ly/2kE4EZ6
- Golbeck JH (2004) Chapter 3: Photosynthetic Reaction Centers in Biophysics. Link: http://bit.ly/2mfHIzv
- Moser CC, Keske JM, Warncke K, Farid SR, Dutton PL (1992) Nature of biological electron transfer. Nature 355: 796-802. Link: https://go.nature.com/2kpNuOG

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
