Open Journal of Analytical and Bioanalytical Chemistry
Identification of Antioxidative Ingredients from Feverfew (Tanacetum Parthenium) Extract Substantially free of Parthenolide and other Alpha-Unsaturated Gamma-Lactones
Fa Zhang*, Jianyun Zhou, Yiqun Shi and Ken Karaisz
Cite this as
Zhang F, Zhou J, Shi Y, Karaisz K (2019) Identification of Antioxidative Ingredients from Feverfew (Tanacetum Parthenium) Extract Substantially free of Parthenolide and other Alpha-Unsaturated Gamma-Lactones. Open J Anal Bioanal Chem 3(1): 076-082. DOI: 10.17352/ojabc.000015Extract of feverfew (Tanacetum Parthenium) has anti-inflammatory effect with various therapeutic benefits. Alpha-unsaturated gamma-lactones including parthenolide were recognized as part of the major active ingredients but with undesirable allergic reactions. In this research paper, feverfew extract substantially free of parthenolide and other alpha-unsaturated gamma-lactones was investigated. We are reporting the identification of more than twenty ingredients from this feverfew extract. The ingredients mainly include caffeoyl derivatives and flavonoids. Four ingredients, caffeic acid methyl ester (2), cynarin (13), 4-methoxyl caffeic acid (22) and 3,4-dimethoxyl caffeic acid (27), were discovered in feverfew for the first time. The identification works were performed primarily using HPLC-UV and HPLC-APCI-MS analyses and comparing with reference compounds. Ingredients caffeic acid (1), caffeic acid methyl ester (2), quercetagetin 3, 6-dimethyl ether (3), apigenin (4), and santin (6), were isolated by semi-preparative HPLC; and their structures were further confirmed by NMR analyses. The application of APPI-MS on the analyses of 1 and 2 enabled successful molecular weight determination and eliminated lingering ambiguity caused by weak signals from APCI-MS detection. The extract of feverfew also demonstrated considerably high antioxidant capacity using a DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging model likely due to its caffeoyl derivatives and flavonoid ingredients with phenolic moieties to contribute to its pharmacological benefits.
Keywords: Feverfew; Antioxidant; Caffeoyl derivatives; Flavonoids
Introduction
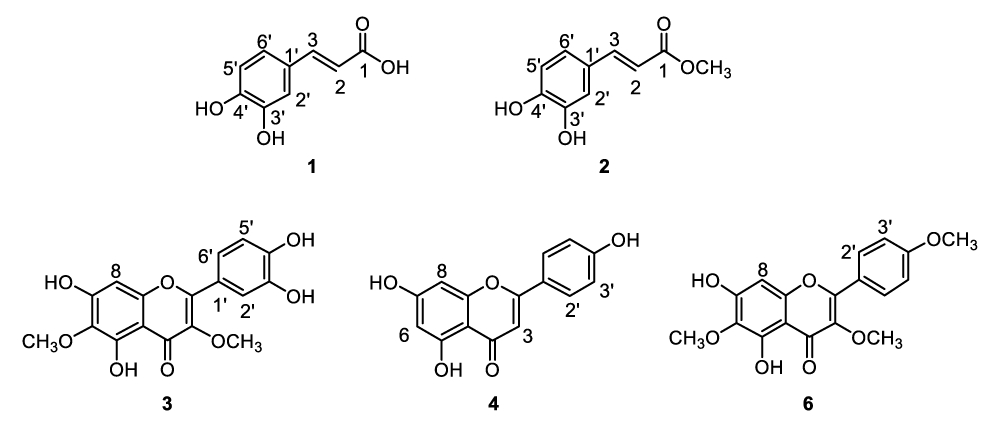
Feverfew (Tanacetum Parthenium) is a medicinal plant that can be found in old gardens or hedgerows. The name of the herb was derived from the Latin word febrifugia, meaning “fever reducer”. It has a long history of usages in traditional and alternative medicines. It was believed to have anti-inflammatory effect and widely used to treat diseases such as fevers, arthritis, digestive problems, migraines and various other conditions. Feverfew is rich in sesquiterpene lactones, essential oils, flavonoids and other minor chemicals [1-5]. Williams, et al., found several flavonoids in feverfew such as tanetin and other flavanol methyl esters, apigenin, luteolin, chrysoeriol as well as their glucuronides and glycosides [6-8]. Other research groups reported the identification of jaceidin, centaureidin, sudachitin, aceronin, nevadensin. and polyphenolic acids [9-12]. The focus initially was on parthenolide which is the predominant sesquiterpene lactone presented in feverfew and has been considered as the compound mainly responsible for the anti-inflammatory effect [1-5,13]. The mechanism of action is presumably through the covalent bonding by Michael addition reaction of alpha-methylenebutyrolactone moiety with cysteine residue of relevant enzymes or proteins [13]. It was also known that allergic reaction to the extract may be caused by sesquiterpene lactones such as alpha-unsaturated-gamma-lactones including parthenolide. Therefore, the therapeutic applications of feverfew extract containing less sesquiterpene lactones were widely sought after [14,15]. Considering the interest generated in feverfew, we initiated a study to profile the structures of the active ingredients in feverfew extract that are substantially free of parthenolide and other alpha-unsaturated gamma-lactones. Twenty-eight ingredients (1-28) in the tested feverfew extract were observed. Two main groups of ingredients, flavonoids and caffeoyl derivatives, were identified. Five of the ingredients, 1, 2, 3, 4 and 6, (Figure 1) were isolated. Modern analytical instrumentation such as HPLC-UV, HPLC-APCI-MS, APPI-MS, and NMR were used to confirm their structures. The structural identification and isolation of caffeic acid methyl ester (2) and discovery of additional caffeoyl derivatives (13, 22, 27) in feverfew extract have not been reported in literature. The findings are a valuable step towards unraveling the entire constituents of feverfew, especially the ones with potential biological functions. It is known that both flavonoids and caffeoyl derivatives are antioxidants with anti-inflammatory and other valuable therapeutic activities [1-12,16-18]. It remains to be seen whether caffeoyl derivatives in feverfew extracts play an independent or synergistic role along with flavonoids and other ingredients. Considerably high antioxidation activity of the feverfew extract that are substantially free of parthenolide and other alpha-unsaturated gamma-lactones was demonstrated by using the DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging model [19].
Experiments
Chemicals: HPLC grade acetonitrile and water were obtained from J.T. Baker. Trifluoroacetic acid (certified) and HPLC grade dimethyl sulfoxide were obtained from Fisher Scientific. The methods for the preparation of feverfew extracts that are substantially free of parthenolide and other alpha-unsaturated gamma-lactones were disclosed in US Patent 6224875B1 [20]. The reference compounds for the confirmation of feverfew ingredients were obtained from multiple sources.
HPLC-UV analyses: The analytical HPLC method (HPLC Method I) used a C18 column (Zorba×SB-C18, 5µm, 25×0.46cm,) at 40°C. A binary gradient elution using mobile phase A and B was conducted (0-20min, linear gradient from 10 to 30% B; 20-25min, linear gradient to 40% B; 25-35min, linear gradient to 60% B; 35-50min, linear gradient to 80% B) at a flow rate of 0.85mL/min. Mobile phase A contained water with 0.01% of trifluoroacetic acid while mobile phase B contained acetonitrile with 0.01% of trifluoroacetic acid. The injection volume was 10µL. An UV PDA detector was used for online UV spectral collection with wavelength at 340nm for chromatographic detection.
Semi preparative HPLC-UV isolation: The semi-preparative HPLC method (HPLC Method II) used a C18 column (Zorba×SB-C18, 5µm, 250×0.94cm,) at 40oC. A binary linear gradient elution using the same mobile phase A and B as HPLC Method I was conducted (0-20min, linear gradient from 10 to 30% B; 20-25min, linear gradient to 40% B; 25-35min, linear gradient to 60% B; 35-50min, linear gradient to 80% B) at a flow rate of 4.0mL/min. The detection wavelength was at 340nm. Injection volume was 0.8mL from a solution of 2grams of extract in 20mL of dimethyl sulfoxide. The collected fractions were transferred to individual flasks and evaporated to dryness using a Brinkmann Evaporator at 45-50°C under vacuum.
HPLC-MS and NMR analyses: HPLC-MS was performed on a Thermo Scientific LXQ HPLC-MS system in APCI or APPI positive ion mode under optimized conditions. HPLC condition was the same as HPLC method I. A Bruker AVII400 FT-NMR Spectrometer was used for NMR analyses with acetone-d6 as the solvent.
Results
HPLC-UV screening
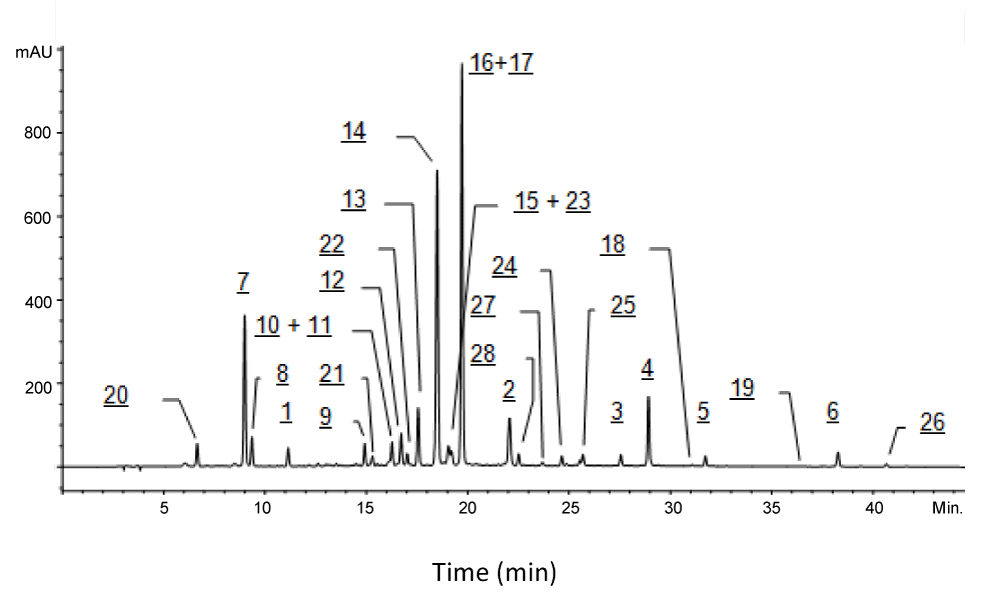
The feverfew extract was dissolved in a mixture of dimethyl sulfoxide and water (1:1) at 1% concentration and analyzed by HPLC-Method I. The resulted chromatogram is shown in Figure 2. Twenty-eight ingredient peaks (1-28) were observed. The major ingredients (>10% per peak area%) of the extract were 16, 17, 14 and 7. The pairs of ingredients, 16 and 17, as well as 10 and 11, were coeluted. Ingredients 15 and 23 were barely separated. Further details of these ingredients are described in following sections.
Identification of caffeoyl derivatives
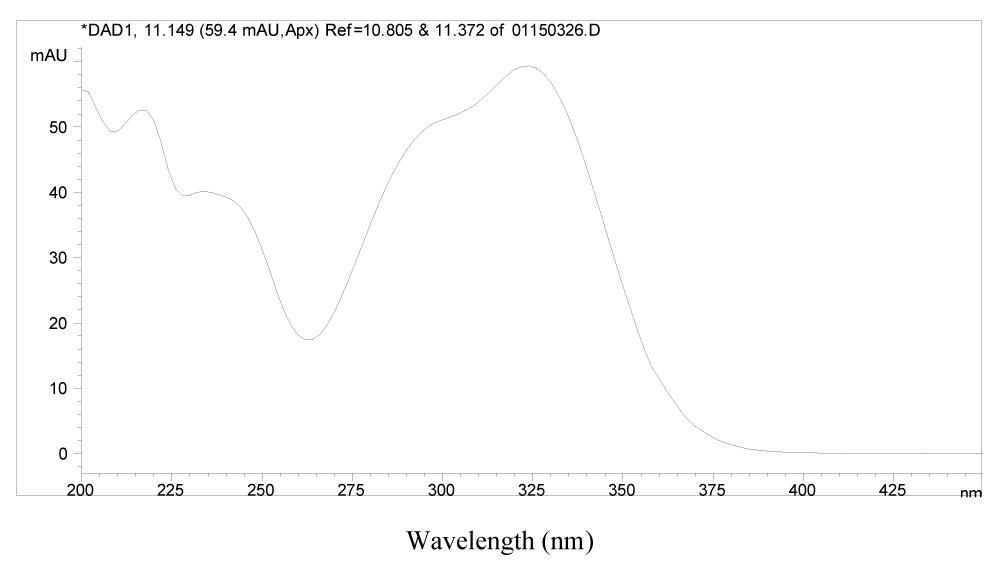
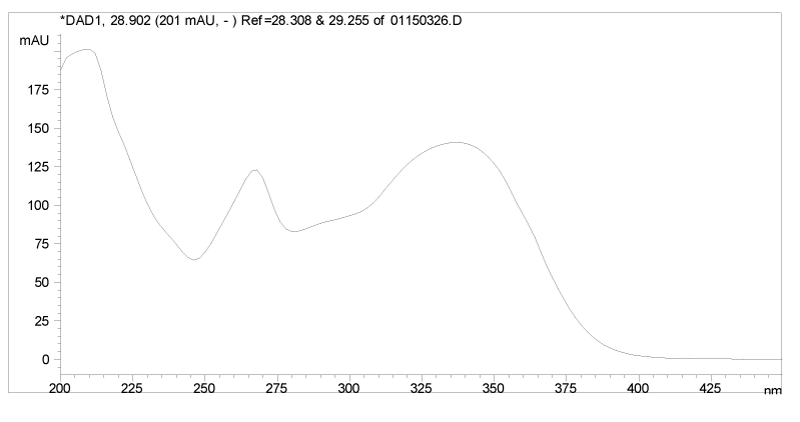
It was found that peaks 1, 2, 8, 20, 21, 22, 23, 27 and 28 all have very similar UV spectra, which suggests that they may be analogues structurally related to each other. Typical UV spectrum of 1 is presented in Figure 3.
Ingredients 1, 2, 8, 20, 21, 22, 23, 27 and 28 did not show reasonable responses to HPLC-APCI-MS detection in positive ion mode. There were no satisfactory mass spectra obtained for molecular weight information. This was likely due to their poor thermal stability at elevated ionization source temperature.
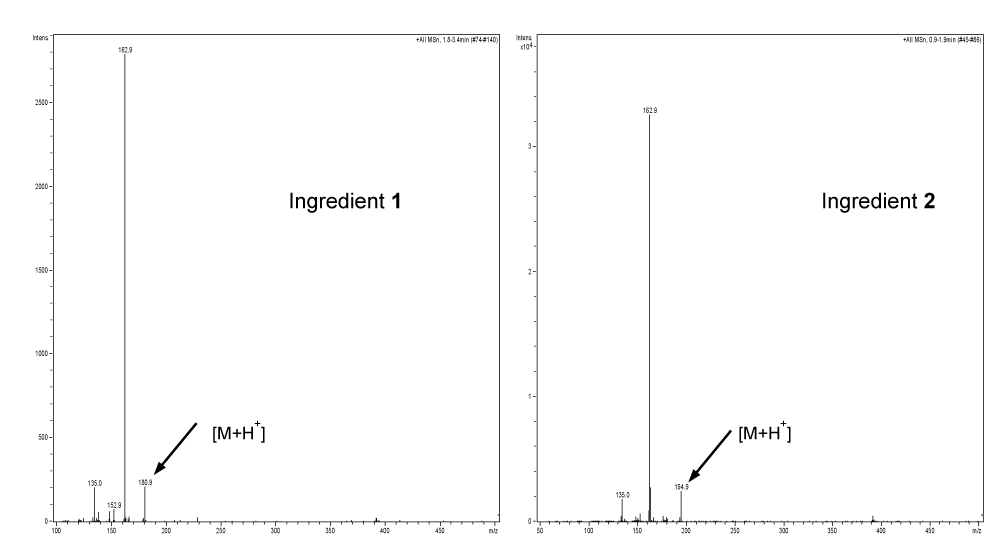
1 and 2 were isolated as pure compounds using semipreparative HPLC Method II as described in section of Experiments. They were analyzed by direct infusion APPI-MS in positive ion mode and NMR. The structures of 1 and 2 were determined as caffeic acid and caffeic acid methyl ester. Their APPI-MS spectra are shown in Figure 4 and their 1H and 13C NMR data are presented in Table 1.
APPI-MS spectra of 1 and 2 exhibited protonated molecular ions at m/z 180 and 194 respectively. 1 and 2 showed very similar 1H NMR or 13C NMR resonance patterns except that 2 exhibited signal for an extra CH3 group. 1 and 2 were also confirmed by comparing their exact same HPLC-UV features with corresponding reference compounds of caffeic acid and caffeic acid methyl ester.
Ingredients 22 and 27 were identified as 4-methoxyl caffeic acid and 3,4-dimethoxyl caffeic acid respectively, solely based on comparing their exact same HPLC retention times and UV spectra with that of corresponding reference compounds.
The absolute structures of ingredients 8, 20, 21, 23, and 28 remain to be established. They are likely caffeoyl derivatives based on their similar UV absorption pattern and poor APCI-MS response feature.
Ingredient 7, 13, 14, 16 exhibited protonated molecular ions at m/z 355, 517, 517, and 517, respectively; they were identified as chlorogenic acid (7), cynarin (13), 3,4-di-O-caffioyl quinic acid (14), and 3,5-di-O-caffioyl quinic acid (16). The structures of 7, 13, 14, and 16 were all confirmed by comparing their exact same HPLC-MS and HPLC-UV features with corresponding reference compounds. Ingredient 16 coeluted with 17 which was identified as apigenin-7-O-glucuronide (see following section). Ingredients 7, 13, 14, and 16 are also caffeoyl derivatives but containing sugar moiety. They showed adequate APCI-MS responses. The possible impacts of sugar moieties on their APCI-MS responses remain to be understood.
Ingredients 1, 7, 14 and 16 were previously observed in feverfew extract [11,12]. However, the detection of ingredients 2, 13, 22 and 27 in feverfew extract were reported for the first time to the best of our knowledge.
The observed ingredients of feverfew extract and HPLC-APCI-MS screening results are summarized in Table 2 along with their relative levels per HPLC-UV chromatographic peak area%.
Identification of flavonoids and parthenolide
Ingredients 3, 4, and 6 exhibited protonated molecular ions at m/z 347, 271, and 345. They were isolated by semi-preparative HPLC as described in the experimental section and analyzed by 1H NMR. The NMR data are presented in Table 3. Ingredients 3, 4, and 6 were determined as quercetagetin 3, 6-dimethyl ether, apigenin, and santin, respectively, which are all flavonoid analogues with similar UV spectral features. As an example, the UV spectrum of apigenin (4) is presented in Figure 5.
The NMR data obtained for 3 and 6 in Table 3 matched well with what published for quercetagetin 3,6-dimethyl ether and santin [7,9]. The structures of 4 and 6 were further confirmed by comparing their exact same HPLC-UV features with that of corresponding reference compounds.
Ingredients 5 and 18 exhibited protonated molecular ions at m/z 361 and 331. The similar UV absorption patterns of these two ingredients to that of ingredients 3, 4 and 6 suggest that they likely contain flavonoid chromophore. Considering the works by Williams, et al. [6-9] and the mass spectrometric and UV data, we speculate that ingredient 5 may be either quercetagetin 3,6,3’-trimethyl ether or quercetagetin 3,6,4’-trimethyl ether; while 18 may be either jaceosidin or 3,6-dimethoxyapigenin. Both 5 and 18 are flavonoids.
Ingredient 9 exhibited protonated molecular ion at m/z 271 which is the same as that of apigenin (4). Ingredient 9 also showed very similar UV spectral features to that of ingredient 4, which suggests ingredient 9 likely to be an isomer of 4. Its absolute structure remains to be established.
Ingredient 10, 11, 12, 15, and 17 exhibited protonated molecular ions at m/z 479, 449, 463, 433, 447, respectively. They were identified as quercetin-7-glucuronide (10), luteolin-7-O-glucuronide (11), luteolin-7-O-glucuronide (12), apigenin-7-O-glucoside (15) and apigenin-7-O-glucuronide (17). They were confirmed by comparing their exact same HPLC-UV and HPLC-MS features with corresponding reference compounds. Ingredients 10 and 11 coeluted. Ingredient 17 coeluted with 16 which was identified as 3,5-di-O-caffeoyl quinic acid as described in previous section. Ingredients 10, 11, 12, 15, and 17 are all flavonoids.
Ingredient 19 was a trace level ingredient (<0.07%) and exhibited protonated molecular ion at m/z 249. It was identified as parthenolide and confirmed by comparing its exact same HPLC-UV and HPLC-MS features with a reference compound.
Ingredient 24 exhibited protonated molecular ion at m/z 461 with typical UV absorption of flavonoid similar to Figure 5. Ingredient 24 was proposed to be apigenin-7-O-β-D-glucuronide methyl ester or its isomer.
Ingredient 25 exhibited ions at m/z 263 and 330 with UV absorption features of flavonoid to suggest that it may be another flavonoid analogue. No satisfactory MS spectrum nor UV spectrum was obtained from ingredient 26 which is a very minor ingredient. The structures of 25 and 26 remain to be determined.
Antioxidant activity of feverfew extract by DPPH free radical scavenging assay
DPPH (1,1-diphenyl-2-picrylhydrazyl) is a stable free radical and has been widely used in evaluating free radical scavenging capability of natural extracts to access their associated antioxidant activity. DPPH exhibits characteristic color of purple with strong absorption at 517nm. Direct colorimetric method has been used to monitor the decrease of DPPH as a result of reaction with antioxidant [19]. However, this direct colorimetric method suffers from interference with tested materials of complicated composition, especially those that have colors. In this report, a reversed-phase HPLC method was developed and used to monitor the change of DPPH during the course of reaction with antioxidants to avoid interference from tested materials or reduced DPPH. The antioxidant activity of feverfew extract along with chlorogenic acid which is a major ingredient of the extract, ascorbic acid, and α-tocopherol were compared. Ascorbic acid and α-tocopherol are well known potent antioxidants.
The HPLC-UV method utilized a reversed-phase C18 column with binary gradient elution and UV detection at 517nm to monitor the levels of DPPH which was incubated with antioxidants at different concentration levels at room temperature for one hour and analyzed subsequently by HPLC. Typical linear relationship between remaining DPPH level and initial concentration of antioxidant can be obtained. A linear regression was utilized to calculate IC50 values, which is the initial concentration of antioxidant needed to reduce DPPH level to 50% of its original concentration. Lower IC50 indicates higher antioxidant activity. The results are listed in Table 4. It indicates that the feverfew extract exhibited considerably high antioxidant activity.
Discussion
Like most botanicals, feverfew is chemically very complex, containing sesquiterpene lactones, flavonoid glycosides, pinenes and other compounds. The specific role that each of these component compounds plays in the biological activity of feverfew, however, is not to date fully understood. However, parthenolide has been thought to be the most active chemical component in feverfew and its anti-inflammatory activities were proposed to be associated with specifically binding to and inhibiting IκB kinase complex (IKK)β which plays an important role in pro-inflammatory cytokine-mediated signaling [1,21]. Chloroform leaf extracts, rich in sesquiterpene lactones including parthenolide, inhibit production of inflammatory prostaglandins in rat and human leukocytes. Inhibition was irreversible and the effect was not caused by cytotoxicity. Studies have shown that lipophilic compounds other than parthenolide may be associated with anti-inflammatory activity, particularly with reducing human neutrophil oxidative burst activity [1,22-24]. Inhibition of prostaglandin synthetase also has been documented for parthenolide [1,25,26]. The anti-inflammatory effects of feverfew could also be caused by a cytotoxic effect. Feverfew extracts were found to inhibit mitogen-induced tritiated thymidine uptake by human peripheral blood mononuclear cells, interleukin-2-induced tritiated thymidine uptake by lymphoblasts, and prostaglandin release by interleukin-1-stimulated synovial cells. Parthenolide was demonstrated to block tritiated thymidine uptake by mitogen-induced human peripheral blood mononuclear cells [1,27]. Parthenolide has been widely used as an active marker for standardization and quality control. Feverfew products are required to contain no less than 0.1% parthenolide in France and 0.2% parthenolide in the US, UK and Canada [28]. Many solvent systems have been reported to extract feverfew for high recovery of parthenolide. It was observed that methanol and 50% ethanol are the two best candidates for highest percentage of parthenolide from feverfew, 50% ethanol is a little better for feverfew extract, while methanol works better for feverfew crude material [28].
However, it is believed that alpha-unsaturated gamma-lactones such as parthenolide could cause many allergic reactions [14,29-33], which arose the interest of using feverfew extract with reduced levels of alpha-unsaturated gamma-lactones including parthenolide for therapeutic applications since feverfew also contain other ingredients such as flavonoids which exhibit anti inflammatory activities [34]. Bombardelli and Morazzoni [20], disclosed a procedure to prepare extracts of feverfew with a reduced content of alpha-unsaturated gamma-lactones, particularly of parthenolide by multistep extraction and elution on basic resins.
This study investigated the ingredients of the aforementioned feverfew extract substantially free of parthenolide and other alpha-unsaturated gamma-lactones. It was confirmed that the level of parthenolide (19) is pretty low (< 0.07%, Table 2) with no other alpha-unsaturated gamma-lactones detected. More than twenty ingredients were characterized to belong mainly to two categories, caffeoyl derivatives and flavonoids. Four caffeoyl derivatives, i.e. caffeic acid methyl ester (2), cynarin (13), 4-methoxyl caffeic acid (22), and 3,4-dimethoxyl caffeic acid (27), were observed for the first time from feverfew extract to the best of our knowledge. The extract was especially enriched in caffeoyl derivatives including chlorogenic acid (7) (12.00%), 3,4-di-O-caffeoyl quinic acid (14) (23.33%), and 3,5-di-O-caffeoyl quinic acid (16) (31.78% together with coeluted apigenin-7-O-glucuronide (11)). It is worthwhile to point out that caffeoyl derivatives appears to be overlooked ingredients in feverfew as reflected in almost all the review articles on feverfew with less attention. Both caffeoyl derivatives and flavonoids are well known antioxidants. Caffeoyl derivatives may even have higher antioxidative potency due to their polyphenolic features, which is consistent with the measured antioxidative activity by DPPH free radical scavenging assay (Table 4) to indicate that the feverfew extract exhibited considerably high antioxidant capacity. Antioxidation is closely associated with anti-inflammatory and other therapeutic benefits. The feverfew extract investigated is substantially free of parthenolide and other alpha-unsaturated gamma-lactones. It is reasonable to expect that the extract still has favorable pharmacological properties together with reduced risks of allergic reactions.
Conclusion
The feverfew extract that was substantially free of parthenolide (< 0.07%) and other alpha-unsaturated gamma-lactones was analyzed with twenty-eight ingredients observed. Th structural identities of the ingredients were elucidated mainly using HPLC-UV, HPLC-MS and comparison with reference compounds. Five ingredients were isolated and characterized further using NMR analyses to determine their structures. Two major groups of ingredients, caffeoyl derivatives and flavonoids as well as parthenolide at trace level, were observed. Four ingredients, i.e. caffeic acid methyl ester (2), cynarin (13), 4-methoxyl caffeic acid (22), and 3,4-dimethoxyl caffeic acid (27), were new findings in feverfew extract to the best of our knowledge. The feverfew extract displayed considerably high antioxidant capacity in a DPPH free radical scavenging assay, which is likely due to the existence of phenolic moieties in caffeoyl derivative and flavonoid ingredients. The extract was especially enriched in caffeoyl derivatives including chlorogenic acid (7) (12.00%), 3,4-di-O-caffeoyl quinic acid (14) (23.33%), and 3,5-di-O-caffeoyl quinic acid (16) (31.78% together with coeluted apigenin-7-O-glucuronide (11)). The antioxidant capacity may potentially contribute to its pharmacological benefits such as anti-inflammatory, inhibition of UV induced matrix metalloproteinase-1 (MMP-1), prevention of smoke-induced loss of thiols, etc. [14,15]. The feverfew extract investigated was substantially free of parthenolide and other alpha-unsaturated gamma-lactones to reduce undesirable allergic reactions
- Pareek A, Suthar M, Rathore GS, Bansal V (2011) Feverfew (Tanacetum Parthenium L.): A systematic review. Pharmacogn Rev 5: 103-110. Link: http://bit.ly/2RJQR1d
- European Medicines Agency Committee on Herbal Medicinal Products (HMPC) (2011) Assessment report on Tanacetum Parthenium (L.) Schulz Bip., herba.
- Knight DW (1995) Feverfew: chemistry and biological activity. Nat Prod Rep 12: 271-276. Link: http://bit.ly/34e13BJ
- Shahhoseini R, Azizi M, Asili J, Moshtaghi N, Samiei L (2019) Comprehensive assessment of phytochemical potential of Tanacetum Parthenium (L.): phenolic compounds, antioxidant activity, essential oil and parthenolide. Journal of Essential Oil Bearing Plants 22: 614-629. Link: http://bit.ly/2RHP3pm
- Pourianezhad F, Tahmasebi S, Abdusi V, Nikfar S, Mirhoseini M (2016) Review on feverfew, a valuable medicinal plant. J HerbMed Pharmacol 5: 45-49. Link: http://bit.ly/2LKTpID
- Williams CA, Hoult JR, Harborne JB, Greenham J, Eagles J (1995) A biologically active lipophilic flavonol from Tanacetum parthenium. Phytochem 38: 267-270. Link: http://bit.ly/2YPYepr
- Williams CA, Harborne JB, Geiger H, Hoult JR (1999) The flavonoids of Tanacetum Parthenium and T. vulgare and their anti-inflammatory properties. Phytochem 51: 417-423. Link: http://bit.ly/2PyRdF4
- Williams CA, Harborne JB, Eagles J (1999) Variations in lipophilic and polar flavonoids in the genus Tanacetum. Phytochem 52: 1301-1306. Link: http://bit.ly/38rKVQp
- Long C, Sauleau P, David B, Lavaud C, Cassabois V, et al. (2003) Bioactive flavonoids of Tanacetum Parthenium revisited. Phytochem 64: 567-569. Link: http://bit.ly/2YGMJk3
- Végh K, Riethmüller E, Hosszú L, Darcsi A, Müller J, et al. (2018) Three newly identified lipophilic flavonoids in Tanacetum Parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J Pharm Biomed Anal 149: 488-493. Link: http://bit.ly/3496XDT
- Wu C, Chen F, Wang X, Wu Y, Dong M, et al. (2007) Identification of antioxidant phenolic compounds in feverfew (Tanacetum Parthenium) by HPLC-ESI-MS/MS and NMR. Phytochem Anal 18: 401-410. Link: http://bit.ly/2YH8IXZ
- Hanganu D, Benedec D, Vlase L, Popica I, Bele C, et al. (2016) Polyphenolic content and antioxidant activity of Chrysanthemum parthenium extract. Farmacia (Bucharest, Romania) 66: 498-501. Link: http://bit.ly/2EbRt7A
- Piela-Smith TH, Liu X (2001) Feverfew extracts and the sesquiterpene lactone parthenolide inhibit intercellular adhesion molecule-1 expression in human synovial fibroblasts, Cellular Immunol 209: 89-96. Link: http://bit.ly/2PAhcfi
- Katharine M, Claude S (2009) Composition containing feverfew extract and use thereof. Patent US 7,547456 B2.
- Katharine M, Claude S, Zhang L, Eisinger M (2004) Composition containing feverfew extract and use thereof. PCT Patent Application No. WO 2004/022028 B2.
- Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang MK, et al. (2009) Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 30: 321-330. Link: http://bit.ly/2RGW9KC
- De P, Baltas M, Bedos-Belval F (2011) Cinnamic acid derivatives as anticancer agents-A review. Curr Med Chem 18: 1672-1703. Link: http://bit.ly/2t8ghuZ
- Fraisse D, Felgines C, Texier O, Lam JL (2011) Caffeoyl derivatives: major antioxidant compounds of some wild herbs of the asteraceae family. Food and Nutrition Sciences 2: 181-192. Liink: http://bit.ly/35d50Yp
- Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181: 1199-1200. Link: https://go.nature.com/2PDMnWZ
- Bombardelli E, Morazzoni P (2001) Tanacetum Parthenium extract and method of obtaining same. US Patent 6224875B1.
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM (2001) The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 8: 759-766. Link: http://bit.ly/36pvear
- Sumner H, Salan U, Knight DW, Hoult JR (1992) Inhibition of 5-lipoxygenase and cyclo-oxygenase in leukocytes by feverfew. Involvement of sesquiterpene lactones and other components. Biochem Pharmacol 43: 2313-2320. Link: http://bit.ly/38vnDZW
- Collier HO, Butt NM, McDonald-Gibson WJ, Saeed SA (1980) Extract of feverfew inhibits prostaglandin biosynthesis Lancet 316: 922-923. Link: http://bit.ly/2E8tzK6
- Brown AM, Edwards CM, Davey MR, Power JB, Lowe KC (1997) Pharmacological activity of feverfew (Tanacetum Parthenium [L.] Schultz-Bip.): Assessment by inhibition of human polymorphonuclear leukocyte chemiluminescence in vitro. J Pharm Pharmacol 49: 558-561. Link: http://bit.ly/2LHiQuz
- Makheja AN, Bailey JM (1982) A platelet phospholipase inhibitor from the medicinal herb feverfew (Tanacetum Parthenium), Prostaglandins Leukot Med 8: 653-660. Link: http://bit.ly/34cdhL8
- Pugh WJ, Sambo K (1988) Prostaglandin synthetase inhibitors in feverfew. J Pharm Pharmacol 40: 743-745. Link: http://bit.ly/36wsGaF
- O'Neill LA, Barrett ML, Lewis GP (1987) Extracts of feverfew inhibit mitogen-induced human peripheral blood mononuclear cell proliferation and cytokine mediated responses: A cytotoxic effect. Br J Clin Pharmacol 23: 81-83. Link: http://bit.ly/2E903nB
- Jin P, Madieh S, Augsburger LL (2008) Selected physical and chemical properties of Feverfew (Tanacetum Parthenium) extracts important for formulated product quality and performance. AAPS PharmSciTech 9: 22-30. Link: http://bit.ly/349u1Tf
- Schulz KH, Hausen BM, Wallhӧfer L, Schmidt-Lӧffler P (1975) Chrysanthemem-Allergy. Arch Derm Forsch 251: 235-244.
- Hausen BM, Schulz KH (1976) Chrysanthemum Allergy. Arch Derm Res 255: 111-121. Link: http://bit.ly/2YGnWMJ
- Orion E, Paulsen E, Andersen KE, Menné T (1998) Comparison of simultaneous patch testing with parthenolide and sesquiterpene lactone mix. Contact Dermatitis 207-208. Link: http://bit.ly/2RKNib4
- Spettoli E, Silvani S, Lucente P, Guerra L, Vincenzi C (1998) Contact dermatitis caused by sesquiterpene lactones. Am J Contact Dermat 9: 49-50. Link: http://bit.ly/349uEMB
- Menagé H, Ross JS, Norris PG, Hawk JL, White IR (1995) Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol 132: 543-547. Link: http://bit.ly/38wQ0XF
- Rateb MEM, El-Gendy ANAM, El-Hawary SS, El-Shamy AM (2007) Phytochemical and biological investigation of Tanacetum Parthenium(L.) cultivated in Egypt. J Med Plant Res 1: 18-26. Link: http://bit.ly/2RJ1a5r
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
