Open Journal of Chemistry
Synthesis and Antimicrobial Evaluation of Some Nitro-Mannich Bases Derived from β-Nitrostyrene
Mardia Telep El-Sayed1*, Eman Kishk2 and El-Sayed Afsah2
2Chemistry Department, Faculty of Science, El-Mansoura University, Mansoura, Egypt
Cite this as
El-Sayed MT, Kishk E, Afsah ES (2015) Synthesis and Antimicrobial Evaluation of Some Nitro-Mannich Bases Derived from β-Nitrostyrene. Open J Chem 1(1): 013-016. DOI: 10.17352/ojc.000003The present work focused on exploring the reactivity of β-nitrostyrene towards Mannich reaction with different approaches. The synthesized nitro-Mannich bases were tested as antimicrobial agents that showed high activity against both gram positive and gram negative bacteria.
Introduction
The chemistry of Mannich bases has been the topic of extensive and rising interest. Mannich bases are potentially resourceful synthetic intermediates, and are used for the synthesis of diversity of carbocyclic, heterocyclic compounds and natural products. There is also escalating interest in Mannich bases due to their broad range of biological and pharmacological activities [1-7]. Mannich reaction (Aminomethylation) consists of the condensation of ammonia, primary or secondary amine, frequently as the hydrochloride, with formaldehyde (or, occasionally other aldehydes) and a substrate (any heterocyclic ketone possessing at least one active hydrogen atom of distinct reactivity. The necessary characteristic of the reaction is the substitute of the active hydrogen atom by an amino methyl or substituted amino methyl group. Mannich reaction using nitroalkanes and associated compounds as substrates are of synthetic significance, and the products are shows potential as biologically active substances. Successful synthesis of nitro Mannich bases derived from nitroalkanes and morpholine or piperidine has been reported [8-10]. It is known that the nitro group is an important constituent of many biologically significant heterocycles, such as the antibiotic drugs nitrofurantoin & nitrofurazone [11].
Materials and Methods
Melting points of the synthesized compounds were determined on Gallenkamp electric melting point apparatus. Elemental analyses were carried out in the Micro analytical unit, Faculty of Science, University of Cairo and in the Micro analytical unit, Faculty of Science, Mansoura University. Infrared spectra were recorded using KBr Wafer technique on a Mattson 5000 FTIR spectrometer. 1H-NMR spectra were recorded on a Varian-Gemini (200 MHz) spectrometer. The 1H-NMR chemical shifts were reported in δ ppm using TMS as internal standard. Mass spectra were carried out on Shimadzu GCMS-QP 1000 EX and HP-5988A spectrometer. The purity of the synthesized compounds was tested by thin layer chromatography (TLC). The preparative (TLC) plate was used to separate the mixtures. Biological activity was performed in plant department faculty of science, Mansoura University.
N-[1-(2-Nitro-1-phenyl)ethyl]-4,4`-trimethylenedipiperidine (5): A mixture of β-nitrostyrene (0.15 g, 0.001 mol) and 4,4`-trimethylenedipiperidine (1 g, 0.005 mol) in ethanol (20 ml) was warmed on a steam bath for five minutes with stirring, then left to cool at room temperature for about one hour then diluted with cold water. The precipitate that formed was filtered off, dried and washed well with hot (ethanol-ethylacetate) to give compound 5 as a pale brown powder m.p. 140 -141ºC; yield 1.5g, (83.5%). IR (cm-1): 3433 (NH), 2923, 2854 (CH. Aliph), 1562, 1367 (NO2), 1H-NMR (200MH, DMSO) δ (ppm): 1.21-1.22 (m, 2H, CH2), 1.24-1.26 (m, 6H, 3CH2), 1.45-1.51(m, 6H, 3CH2), 1.52 (t, 1H, J= 2.4Hz, CH), 1.56 (t, 1H, J= 2.7 Hz, CH), 1.91-1.93 (m, 1H, CH), 2.54-2.57 (m, 4H, 2CH2, CH2NHCH2), 2.84-2.86(m, 4H, 2CH2, CH2NCH2), 3.75 (t, 1H, J=2.4 Hz, NCHCH2NO2), 5.02 (dd, 2H, J= 2.3, 5.7Hz, CH2NO2), 7.21-7.25(t, 2H, J= 7.5Hz, ArH), 7.41-7.43 (T, 2H, J=8 Hz, ArH), 13C-NMR (100MH, DMSO) δ (ppm): 19.80, 20.21, 25.20, 25.52, 25.78, 32.31, 32.58, 33.32, 33.31, 44.15, 44.20, 48.12, 48.25, 55.89, 87.23, 124.00, 127.22, 128.15, 129.26, 130.12, 138.43. Elemental Analysis: Calculated for C21H33N3O2 C, 70.16 %; H, 9.25 %; N, 11.69 % (359.50) Found C, 70.12 %; H, 9.22 %; N, 11.70 %.
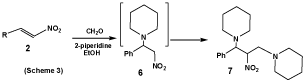
1,3-Di(N-piperidino)-2-nitro-3-phenylpropane (7): A mixture of β-nitrostyrene (0.3 g, 0.002 mol), piperidine (0.34g, 0.004mol) and formalin solution (0.16 ml, 0.002 mol) in ethanol (15 ml) was warmed for 10 minutes on a steam bath, then the mixture stand over 48 hours. The product that formed was filtered off, dried, and washed two times with hot ethanol to give compound 7 as a pale brown fine powder, m.p. 170-171ºC, yield 0.55g, (82.9%). IR (cm-1): 2926, 2855 (CH. Aliph), 1555, 1365 (NO2). MS: m/z (%): 333 (M++2) (8.5), 254(8.1), 247(14.6), 233(5.5), 174(3.2), 156(5.2), 98(15.8), 91(ph-CH2) (100 base peak) 90 (ph-CH) (17.1), 84(18.9), 77(88.0). 1H-NMR (200MH, DMSO) δ (ppm): 1.42-1.46 (m, 4H, 2CH2), 1.54-1.58 (m, 4H, 2CH2), 1.61-1.64 (m, 4H, 2CH2), 2.43-2.48 (t, 8H, J= 2.4, 3.5Hz, 4CH2, CH2NCH2), 3.02-3.04 (m, 2H, NCH2CHNO2), 3.91 (d, 1H, J=3.4Hz, NCHCHNO2), 4.51-4.55 (m, 1H, CHNO2), 7.28-7.29 (m, 1H, ArH), 7.31-7.34 (m, 2H, ArH), 7.42-7.45 (m, 2H, ArH). 13C-NMR (100MH, DMSO) δ (ppm): 24.30, 24.56, 25.80, 25.93, 26.22, 26.25, 45.14, 45.20, 50.00, 52.40, 53.11, 57.18, 87.81, 126.18, 127.30, 128.12, 129.11, 130.14, 140.01. Elemental Analysis: Calculated for C19H29N3O2 C, 68.85%; H, 8.82%; N, 12.68 % (331.45) Found, C, 68.87 %; H, 8.80 %; N, 12.71 %.
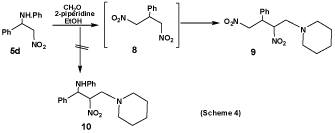
1-(N-Piperidino)-2,4-dinitro-3-phenylbutane (9): A mixture of 5d (0.4 g, 0.002 mol), piperidine (0.4 ml, 0.004 mol) and formalin solution (0.32 ml, 0.004 mol) in ethanol (20 ml) was stirred under cooling for 1 hour, and then the mixture left over 48 hours at room temperature. The product that appeared was filtered off, dried, and crystallized from (ethanol-benzene) to give compound 9 as a white yellowish powder, m.p. 174-175 ºC; yield 0.4g, (63.6 %). IR (cm-1): 3452 (OH hydrate), 2955, 2812(CH. Aliph), 1554, 1342 (NO2). MS (m/z) (%): 316(M+) (1.0), 317(M++1) (0.4), 315(M+-1) (3.5), 314(M+-2) (20.1), 217(12.9), 158 (1.1), 98(100 base peak), 84(3.5), 77(5.7).). 1H-NMR (200MH, DMSO) δ (ppm): 1.51-1.54 (m, 4H, 2CH2), 1.60-1.62 (m, 2H, CH2), 2.39-2.42(M, 4H, 2CH2, CH2NCH2), 2.78-2.79(M, 1H, CHPh), 3.01-3.03 (m, 2H, CH2, NCH2CH), 4.51-4.52(M, 1H, CHNO2), 4.92-4.95(M, 2H, CH2NO2), 7.21-7.24 (m, 1H, ArH), 7.31-7.35 (m, 2H, ArH), 7.39-7.41(m, 2H, ArH). 13C-NMR (100MH, DMSO) δ (ppm): 23.51, 24.12, 24.81, 30.22, 51.25, 54.15, 55.00, 75.43, 88.01, 125.12, 127.82, 127.99, 129.02, 129.11, and 137.24. Elemental Analysis: Calculated for C15H21N3O4. ½ H2O C, 56.94 %; H, 6.69%; N, 13.29 % (316.30) Found C, 56.91%; H, 6.70%; N, 13.27 %.
Anti-microbial evaluation
The antimicrobial activity of the prepared new nitro-Mannich bases was performed using the Cup-diffusion technique as described by Arret et al. [12] and code of Federal Regulations [13].
Cup-diffusion technique
In this experiment, the pores are made using a sterile cork poorer in the solidified agar medium and aliquot of 200 μL of the tested substrates (0.1gm in 0.5ml of dimethyl sulphoxide, (DMSO) which is biologically inactive) is placed in each pore in the nutrient agar medium, on which a culture of test bacteria has been spread to produce uniform and homogeneous growth. The mean values of area of inhibition were measured and expressed in (cm2), after incubation at 32ºC for 24hrs. The tested bacteria was the following two types: Bacillus Subtillus, as an example for Gram-positive bacteria and Escherishia coli, as an example for Gram negative bacteria, where the result ware collected in Table 1. It has been found that the new nitro compounds exhibit different patterns of antimicrobial activity against the tested bacteria Bacillus subitillus and Escherishia coli, as shown in Table 1.
Results and Discussion
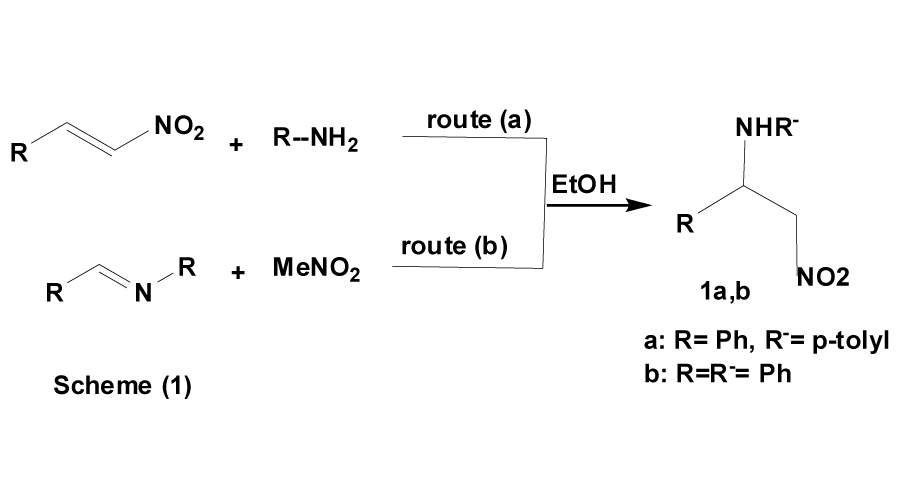
The addition of primary or secondary amines to activated double bonds (e.g. α,β-unsaturated ketones), has been successfully used as a route to special types of Mannich bases [14,15]. In particular, it has been reported earlier [16] that the nitro-base (1a), derived from nitromethane, benzaldehyde and p-toluidine, has been obtained by treating β-nitrostyrene with p-toluidine; route (a) (Scheme 1).
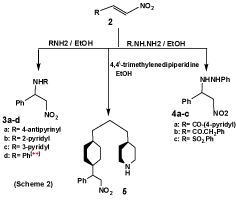
Nitro-bases of the type 1b have been also obtained by addition of nitromethane to aldimines [17], route (b). These reactions constitute a useful synthetic approach to nitro-bases (1), derived from aromatic aldehydes and primary aromatic amines, that dose not undergo Mannich reaction. In the present work, it has been found that the addition of 4-aminoantipyrine, 2-aminopyridine and 3-aminopyridine to β-nitrostyrene (2) is a valuable alternative to Mannich reaction with nitromethane. Therefore, 1-(4-antipyrinylamino)-2-nitro-1-phenylethane (3a), 1-(2-pyridylamino) and 1-(3-pyridylamino)-2-nitro-1-phenylethane (3b,c) were obtained. The formation of compounds 3a-d has been found to take place smoothly and with good yields. This reaction has been extended to prepare a series of 1-acylhydrazino-2-nitro-1-phenylethanes (4a-c), by treating 2 with isoniocotinic hydrazide, phenylacetic hydrazide and benzenesulfonyl hydrazide, respectively. A similar reaction takes place with 4,4`-trimethylenedipiperidine yielding N-[1-(2-nitro-1-phenyl)ethyl]-4,4`-trimethylenedipiperidine (5) (Scheme 2).
The analytical and spectral data are consistent with the structures proposed for compounds 3a-d, 4a-e and 5. In connection with the present study, it has been found that treatment of β-nitrostyrene (2) with 2 moles of piperidine and formaldehyde lead to the formation of 1,3-di(N-piperidino)-2-nitro-3-phenylpropane (7). Obviously, compound 7 was formed via a Mannich reaction of the intermediate nitro-base (6) with piperidine and formaldehyde. The structure of compound 7 was supported by analytical and spectral data (Scheme 3).
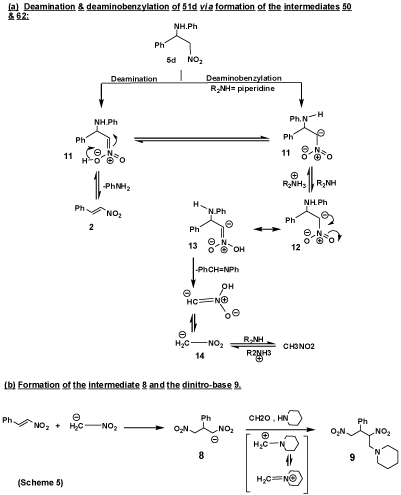
Whereas, an attempted Mannich reaction of 5d, which was prepared according to an earlier report [17], with piperidine and formaldehyde gave a product which was identified as 1-(N-piperidino)-2,4-dinitro-3-phenylbutane (9). The analytical and mass spectral data of 9 were consistent with the molecular formula C15H21N3O4, its mass spectrum showed the molecular ion at m/z 316 (M+) (11%) and at 317 (M++1) (0.4%), 315(M+-1) (3.5%) and 314(M+-2) (20.1%). The base peak at m/z 98(100%) is due to the N-pipridinomethyl fragment. The peak at m/z 217(12.9%) corresponds to [M+-(N-piperidinomethyl)+1)].
Proposed mechanism for formation of compound 9
It is believed that compound 9 is formed by a mechanism which involves the formation of 1,3-dinitro-2-phenylpropane (8), (Scheme 4), as the main intermediate in the reaction sequence. Its formation from 5d may be rationalized on the basis of a mechanism involving both deamination and deaminobenzylation of 11, to give β-nitrostyrene (2) and the nitromethyl anion 14, respectively. Further reaction of 2 with 14 lead to 8, which undergo Mannich reaction with piperidine and formaldehyde to give the end product 9. The deamination of 5d is in line with many reported cases in which free Mannich bases undergo deamination spontaneously [18,19], to give unsaturated products. On the other hand, the tendency of 5d to undergo deaminobenzylation is enhanced by the presence of the strong base piperidine (pkb 2.80), which abstracts a proton from 5d to give the carbanion (11), which is stabilized by delocalization of its charge (12 ↔ 13. The elimination of the nitromethyl anion (14) from 13 is believed to be due to incipient transfer of proton. Addition of the anion 14 to 2 lead to the intermediate 8, which reacts with piperidine and formaldehyde to give 9 as the end product. The cleavage of the phenylaminobenzyl residue from 5d and formation of 8 as intermediate is in line with the reported deaminobenzylation [19] and deaminomethylation [20], of Mannich bases in basic medium with subsequent formation of benzylidene or methylenebis-derivatives (Scheme 5).
Antimicrobial results
The antimicrobial activity test for some of the prepared nitro Mannich bases were listed in Table 1. The tested bacteria was the following two types: Bacillus Subtillus, as an example for Gram-positive bacteria and Escherishia coli, as an example for Gram negative bacteria, where the result ware collected in Table 1. It has been found that the new nitro compounds exhibit different patterns of antimicrobial activity against the tested bacteria Bacillus subitillus and Escherishia coli, as shown in Table 1. Generally, the inhibitory effect of some of these nitro compounds on Gram (-ve) bacteria was the same or more or less potent than their effect on Gram (+ve) bacteria. These data showed that, the highly active substances against gram positive and gram negative bacteria are: 1-(N-piperidino)-2,4-dinitro-3-phenylbutane (9); and 1,3-di(N-piperidino)-2-nitro-3-phenylpropane (7), with zone of inhibition in (cm2) against Bacillus subitillus 16.24 cm2 and 15.19 cm2 respectivily, and zone of inhibition in (cm2) against Escherishia coli 14.70 and 15.19 respectivily. Compounds 1-(4-antipyrinylamino)-2-nitro-1-phenylethane (3a); 1-(2-pyridylamino)-2-nitro-1-phenylethane (3b); 1-(3-pyridylamino)-2-nitro-1-phenylethane (3c); and 1-acylhydrazino-2-nitro-1-phenylethane (4a-c) as six different prouducts were identified as the same acntimicrobial activity against both gram positive and gram negative bacteria with zone of inhibition 12.56 cm2. On the other hand, compound N-[1-(2-nitro-1-phenyl)ethyl]-4,4`-trimethylene dipiperidine (5) showed law or inactive antimacrobial activity.
Conclusion
In this research some interested nitro-Mannich bases were synthesized and tested as antimicrobial agents against bacillus subitillus as gram positive bacteria and escherishia coli as gram negative. The tested nitro-Mannich bases indicated as active antimicrobial agents. As general, the inhibitory effect of some of these nitro compounds on Gram (-ve) bacteria was the same or more or less potent than their effect on Gram (+ve) bacteria.
Authors acknowledge the Applied Organic Chemistry Department, National Research Centre of Egypt and the Chemistry Department Faculty of Science Al Mansoura University for the financial support.
- Tramontini M, Synthesis, El Sayed MT (2015) Strategies for the Synthesis of Mannich Bases, first edition. Lap Lambert, Academic Publishing ISBN: 9783659750847.
- El-Sayed TM, Mahmoud K, Hilgeroth A (2013) Synthesis of β-Nitroamines via Classical Mannich and Aza-Henry Reactions. Current Organic Chemistry 17: 1200.
- Tramontini M, Angiolini L (1990) Further advances in the chemistry of mannich bases. Tetrahedron 46: 1791-1837.
- El-Sayed TM, Abbas M, Hilgeroh A (2011) Efficient One-Pot Formation of Substituted γ-Amino Acids. Letters in Organic Chemistry 8: 320-324.
- Ha HJ, Lee WK (2002) Synthetic applications of Lewis acid-induced n-Methyleneamine equivalents Heterocycles 57: 1525-1538.
- Gul H, Qjanen T, Hanninen O, El Sayed MT, Hussein HAR, et al. (2015) Synthesis and antiproliferative activity of some novel indole Mannich bases. Adv Mod Oncol Res 1.
- Lóránd T, Kocsis B, Sohár P, Nagy G, József P, et al. (2002) Synthesis and antibacterial activity of fused Mannich ketones.Eur J Med Chem 37: 803-812.
- Belikov VM, Belokon Yu N, Dolgaya MM, Martinkova NS (1970) The kinetics and mechanism of Mannich base dissociation in aqueous buffers. Tetrahedron 26: 1199-1216.
- Zief M, Mason P (1943) Reactions of Morpholinemethanol With Compounds Containing Active Hydrogen Atoms. J Org Chem 8: 1-6.
- Johnson HG (1946) Reaction of Aliphatic Amines with Formaldehyde and Nitroparaffins. II. Secondary Amines. J Amer Chem Soc 68; 12-14.
- The Merk Index of Chemicals and Drugs, 9th ed, items No. 6421 and 6422.
- Arret B, Johnson OP, Kirshbaur A (1971) Outline of details for microbiological assays of antibiotics: Second revision. J pharm Sci 60: 1689-1694.
- Code of Federal regulations title 21, Food and Drugs, part 436, subpart D 1983, Microbiological assay method P. 242-259. Office of Federal Register, National Archives and Records Service, Administration, Washington D.C.
- Afsah EM, Hammouda M, khalifa MM, Naturforsch Z (1990) 45b: 1055.
- Afsah EM, Amer FA, Etman H (1979) ibid 34b: 502.
- Worrall DE, Amer J (1927) Chem Soc (19270 49: 1598.
- Leonard NJ, Leubner GW, Burk EH (1950) Reactions of Nitroparaffins with imines and pseudo bases. J Org Chem 15: 979-983.
- Blicke FF (1942) Org Reaet 1: 303.
- Afsah EM, Etman HA, Hamama WS, Sayed-Ahmed AF (1998) Boll Chim Farmaceutico 137: 244.
- Arend M, Westerman B, Risch N (1998) Modern variants of the Mannich reaction. Angew Chem Int Ed Engl 37: 1045.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
