A proposal on a catalyst for the reaction methane + water => methanol + hydrogen
Department of Chemical Engineering, University of Lund, Box 124 SE 221 00 Lund, Sweden
Author and article information
Cite this as
Larsson R (2021) A proposal on a catalyst for the reaction methane + water => methanol + hydrogen. Open J Chem. 2021; 7(1): 001-003. Available from: 10.17352/ojc.000022
Copyright License
© 2021 Larsson R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Based on the concepts and vocabulary of the SET model of catalysis a discussion is performed on what properties should characterize a catalyst promoting a reaction such as the one in the title, i.e., the production of methanol from methane in a non-oxidative environment. It is found that the n1 vibration of water (3652 cm-1 ) and the ny4 vibration of methane (1306 cm-1) interact in resonance.
This means that 1306/3652 = 0.3576 whereas 5:14 = 0.3571. The difference between these two ratios is thus 0.0005. One notes that both frequency-values contain a factor of 1306/5 = 261 cm-1. The conclusion is that also the catalyst must take part in and promote that resonance, containing the same factor, 261 cm-1.
A reaction turning methane into methanol seems to be of great technological and economic importance [1-3]. As methane is a strong “greenhouse - gas” it is of great importance to avoid increasing its concentration in the present atmosphere. The above-mentioned reactions are basically turned out as an oxidation, primarily of the catalyst system using molecular oxygen [2]. However, from the perspective of previous work on the origin of life [4-6], it is interesting to ask: what catalysts might have been operating in prebiotic times when the atmosphere was of a purely reductive kind. We will here exemplify this search on the reaction:
CH4 + H2O ➔ CH3OH + H2 (1)
Selective Energy Transfer or “SET”
The SET model of catalysis [7] relates to an observation [8] that the energies of activation for one and the same reaction but with slight differences in the conditions (e.g. different support of the catalyst) changed in a step-wise manner.
The ‘step’ could be identified as the vibrational quantum of the vibration of the reactant that made the reaction occur and, likewise, of the vibration of the catalyst system. When these two quanta are identical to size, resonance between catalyst and reactant occurs, the frequencies of the two vibrations mentioned are equal and energy can easily be transferred from catalyst to reactant.
It should be noted that not only a 1:1 ratio between catalyst and reactant vibrations gives a resonance condition, but also such ratios as, e.g., 1:2, 2:1, and 2:3 are found.
The SET model has been used successfully to describe the so called ‘compensation effect’ [9]. Further details of SET are given in a review paper [10].
Conventionally, a catalyst is supposed to gather the reactants in spe, on the surface or otherwise, and by this intimate contact the molecules will influence each other so that a reaction comes about.
From the findings in Ref [8], indicating that one special frequency of the reacting molecule builds up the energy of activation, however, we abandoned the above-mentioned concept , in favour of a view that stressed the importance of interactions between a special vibration of the catalyst and a similar vibration within the reactant in spe. If the frequencies of these vibrations were of exactly the same size, a state of resonance between the said vibrations existed and energy could be transferred from catalyst to the vibration of the reacting molecule, speeding up the reaction as a good catalyst should do. Because of this process, the new model of catalysis was named by M Borowiak [11] as “Selective Energy Transfer” or SET.
One might note that the phrase above “of exactly the same size” must be treated carefully. The ratio between the two frequencies must not necessarily be 1:1, it might be 1:2, 1:3 or 2:1 etc.
An example of this is found in Ref [4] treating a condensation of two amino acids. Here we found that the out-of-plane vibration of one of the amino groups of a metal chelate (1058 cm-1 ) was in good resonance with the dominating vibration of the catalyst, COS (2079 cm-1 ) , i.e. a 1:2 type of resonance. Thus the out-of-plane vibration could be strongly excited by the transfer of energy from the catalyst vibrating at 2079 cm-1. When the energy of activation was reached, both other NH2 hydrogen atoms were greatly outside of the original NH2 plane and one of those could attack the carboxylic OH group of the other (nearby) amino acid thus forming water and a peptide bond (C=O)-NH).
Vibrations of methane and water
It is reasonable to consider reaction (1) taking place between components in their gaseous forms. In Table 1, therefore, a collation of vibrational data of methane and water is given. The data are from Herzberg [12] and for CH4 only those vibrations that are IR active are recorded as they might infer a breakage of the bonds in the molecule.
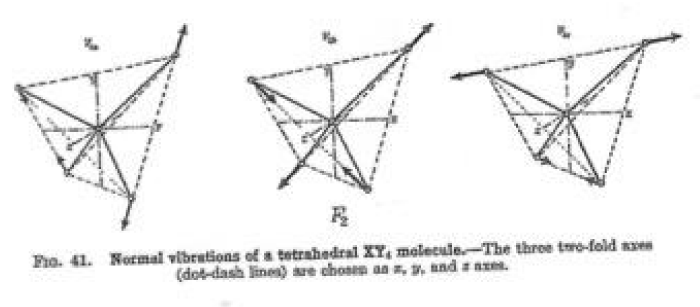
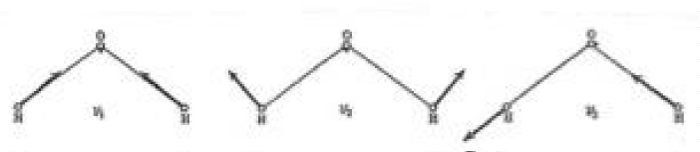
One notes from Tables 1a,1b the differences between the ratios described in the tables and the ratios of integers designed as closely as possible to the expected values for the systems studied differ considerable. The least one, row 3 in Table 1b, concerns the vibration η4 of CH4 and n1 of H2O and indicates that those two vibrations are in resonance to each other. The physical form of these vibrations are given in Figures 1,2.
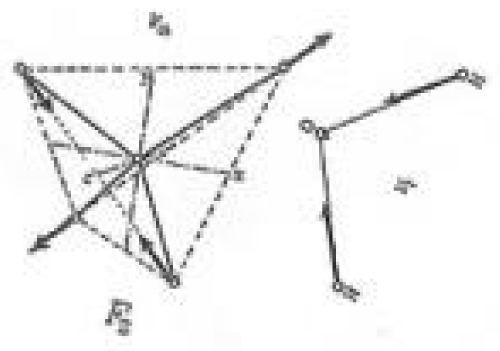
From Figure 3 one can note that the water oxygen atom is expelling the hydrogen atom in the upper right corner of the tetrahedron at the same time as the uppermost (according to the graph) hydrogen atom of water is approaching the expelled H atom , forming a covalent bond (H2).
To the Editor: The vibration of the water molecule ought to be written in phase with the H-C-H vibration of CH4, so that the two H atoms are seen to be moving outwards in the picture. (I.e. the arrows of the smaller part of Figure 3 sould point in the opposite direction).
The catalyst
If the reaction, dealt with above, should be realized a catalyst is needed, having the following characteristics:
1. It must be a solid phase material, so that the gas-formed reactants as well as the products (methanol and hydrogen) might be easily separated from the catalyst.
2. It should have a vibration frequency of one of the reactants, preferably that of methane (1306cm-1 ) so that energy can be spread from the catalyst to the first reactant (methane) via resonance, and further to the second reactant (water) also via vibrational resonance.
3. The catalyst must have a strong absorption of light at the frequency selected. Hence it has also a strong emission of light, making possible transfer of energy over a distance.
4. The frequency of the proposed catalyst must not necessarily be 1306 cm-1, it might be 1306 / 2 = 653 cm-1, or 1306 /3 = 435 cm-1 or even 1306 / 5 = 261 cm-1.
The latter figure is interesting, because 261 x 14 = 3654 cm-1
and the vibration of water (η1 = 3652 cm-1; Table 1 b) that indicates resonance (chapt. 3) between the vibrations of the two reactants.
It might be so that one can regard 261 cm-1 as a measure of the “energy quantum” or “critical vibration” [7,8] that builds the activation energy. However, data on activation energies for the imagined reaction is, of course, not available.
Conclusions
The concept of resonance as a way to transfer energy from catalyst to reactants can be used also to spot possible substances catalytic ability. The treatment is based on the fact that the η1 of water and η4 of methane seem to be in resonance.
- Gesser HG, Hunteer NH, Prakash ChB (1985) The Direct Conversion of Methane to Methanol by Controlled Oxidation. Chem Rev 85: 235-244. Link: http://bit.ly/2Lip1ba
- Mahyuddin MH, Shiota Y, Yoshizawa K (2019) Methane Selective Oxidation to Methanol by Metal-Exchanged Zeolites: A Review of Active Sites and Their Reactivity. Catal Sci Technol 9: 1744-1768. Link: http://rsc.li/38cUB38
- Sushkevich VL, Palagin D, Ranocchiari M, van Bokhoven JA (2017) Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356: 523-527. Link: http://bit.ly/3pM9NtI
- Larsson R, Malek A, Odenbrand I (2019) The transformation by catalysis of prebiotic systems to useful biochemicals: A perspective based on IR spectroscopy of the primary chemicals. I. The Synthesis of Peptides by the Condensation of Amino Acids. Appl Sci 10: 928. Link: http://bit.ly/3n8OIbf
- Larsson R, Malek A, Odenbrand I (2020) The transformation by catalysis of prebiotic systems to useful biochemicals: A perspective based on IR spectroscopy of the primary chemicals. II. Catalysis and the Building of RNA. Appl Sci 10: 4712. Link: http://bit.ly/3nbbnnp
- Larsson R, Malek A, Odenbrand I (2019) The transformation by catalysis of prebiotic systems to useful biochemicals: A perspective based on IR spectroscopy of the primary chemicals. III. In preparation.
- Larssson R (1989) A Model of Selective Energy Transfer at the Active Site of the Catalyst. J Mol Catal 55: 70-83. Link: http://bit.ly/352R2dG
- Larsson R (1987) On the Stepwise Change of the Activation Energy of Catalytic Reactions. Z Phys Chemie Leipzig 268: 721-732. Link: http://bit.ly/38VAxRL
- Keane MA, Larsson R (2007) Isokinetic behaviour in gas phase catalytic hydrodechlorination of chlorobenzene over supported nickel. J Mol Catal A Chem 268: 87-94. Link: http://bit.ly/3b4fLSF
- Larsson R (2013) Concluding remarks on the theory of selective energy transfer and exemplification on a zeolite kinetic study. Monatsh Chem Chemical Monthly 144: 21-28. Link: http://bit.ly/2mYqnvl
- Larsson R, Borowiak M (2001) A Study on Energy and Time description of the Catalytic act with Selective Energy Transfer and Impulse Oscillation Models. J Mol Catal A: Chemical 166: 39-445. Link: http://bit.ly/3hF0o4l .
- Herzberg G (1962) Molecular Spectra and Molecular Structure. II. Infrared and Raman Spectra of Polyatomic Molecules. D van Nostrand Company, Inc. Princeton. Link:
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
