Open Journal of Chemistry
Enhancing future technologies: Sol-Gel synthesis of Sr0.6Ag0.4MnO3 manganite perovskite
Faouzia Tayari1*, Kais Iben Nassar1,2 and Majdi Benamara1,3
2CICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, Santiago University Campus, Aveiro, Portugal
3Laboratory for Building Energy Materials and Components, Swiss Federal Laboratories for Materials Science and Technology (Empa), Überlandstrasse 129, 8600 Dübendorf, Switzerland
Cite this as
Tayari F, Nassar KI, Benamara M (2024) Enhancing future technologies: Sol-Gel synthesis of Sr0.6Ag0.4MnO3 manganite perovskite. Open Journal of Chemistry 10(1): 044-046. DOI: 10.17352/ojc.000038Copyright
© 2024 Tayari F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The research successfully produced Sr0.6Ag0.4MnO3, a silver strontium manganite with the desired perovskite crystal structure, using the sol-gel technique. Extensive analysis revealed its notable characteristics, indicating potential uses across various fields. X-ray diffraction showed the compound's tetragonal structure at room temperature, affirming its stability. Morphological and chemical assessments confirmed the material's consistency and evenness, with crystallites averaging 27 nm (from XRD) and 90 nm (from SEM). The material displayed a ferro-paramagnetic transition at 375 K, suggesting suitability for magnetic applications, alongside a slight drop in electrical resistance under a magnetic field, hinting at potential magnetoresistive properties for electronic devices. In terms of dielectric properties, particularly at low frequencies, the material demonstrated a high dielectric constant and low tangent loss, indicating its potential for electrical components. Overall, these findings position Sr0.6Ag0.4MnO3 as a versatile material with promising applications in magnetism, electronics, and electrical components.
Introduction
Perovskite materials, known for their versatile properties, have attracted scientific interest in diverse technological applications. Among them, SrMnO3 (SMO) stands out for its unique electrical and magnetic behaviors. Researchers are exploring perovskite doping, including substituting ions like Ag into SMO, to enhance its properties further. SMO, with its ABO3 crystal structure, displays paraelectric behavior and antiferromagnetic ordering. Its response to mechanical strain is particularly intriguing, as controlled strain can induce ferroelectric properties and alter its magnetic behavior [1-3]. This study focuses on Sr0.6Ag0.4MnO3 nanoparticles, aiming to understand how Ag doping affects their structural, electrical, dielectric, and magnetic properties. Limited research exists on these nanoparticles, especially regarding the influence of doping concentration. The study reports the hydrothermal synthesis of Sr0.6Ag0.4MnO3 nanoparticles, highlighting their single-phase structure, uniform distribution, high electrical conductivity, and enhanced ferromagnetic behavior at room temperature.
Experimental work
The synthesis of Sr0.6Ag0.4MnO3 nanoparticles involved a meticulous sol-gel process with precursors including strontium nitrate, silver nitrate, and manganese II chloride tetrahydrate. The mixture underwent dissolution in nitric acid, heating, gel formation, and calcination, followed by crushing and pressing into circular pellets at varying temperatures. Characterization of the synthesized samples utilized advanced techniques such as X-ray diffraction, scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray analysis, magnetometry, and impedance analysis to explore structural, morphological, compositional, magnetic, and electrical characteristics comprehensively [4].
Results and discussion
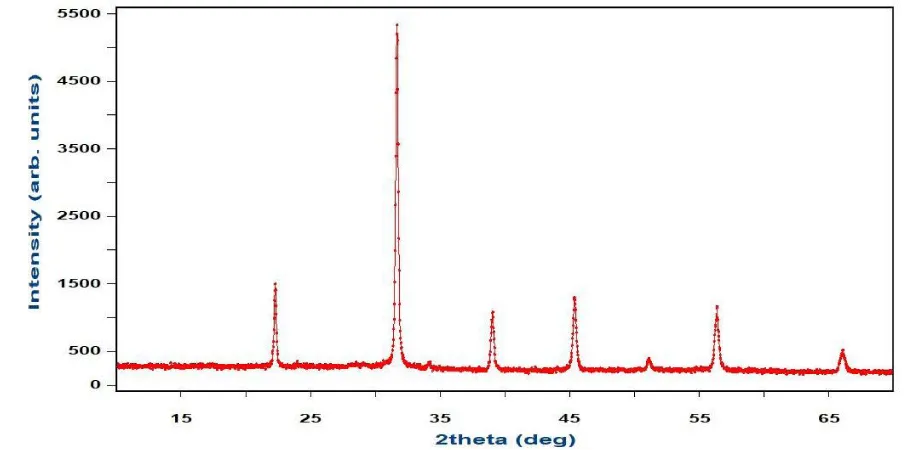
The X-ray Diffraction (XRD) patterns of the Sr0.6Ag0.4MnO3 sample displayed the crystalline structure, confirming its perovskite nature (Figure 1). Analysis of the powder XRD pattern revealed diffraction peaks indexed within the tetragonal perovskite structure with the space group I4/mcm. Utilizing the Rietveld method with the Fullprof program indicated no secondary phases, affirming the material's single-phase nature [5-8]. The agreement between calculated and measured intensities suggested robust crystallization. Additionally, Scherrer’s equation was employed to determine the crystallite size, yielding a value of 27 nm.
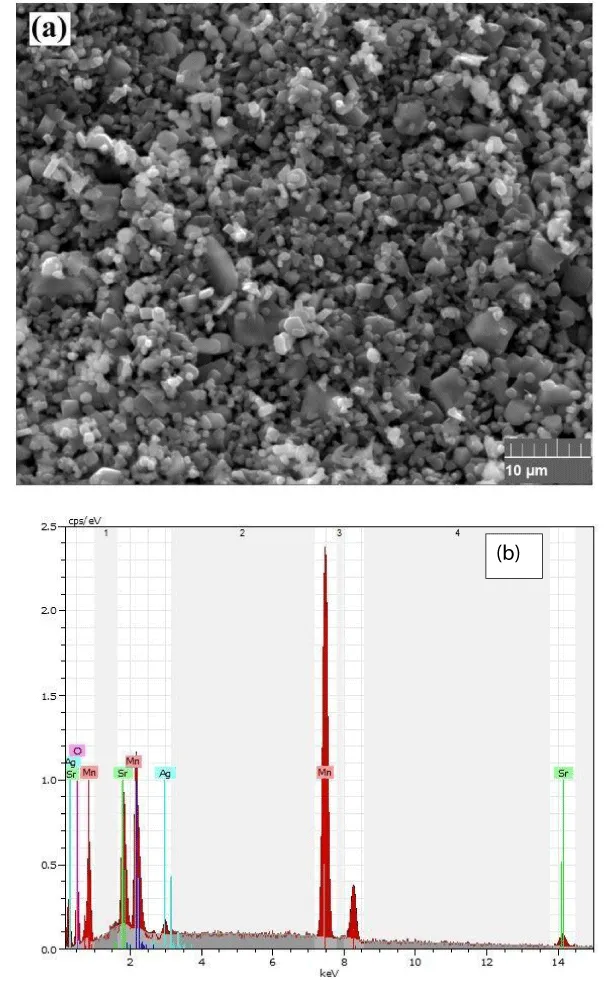
The SEM analysis of annealed Sr0.6Ag0.4MnO3 nanoparticles at 1000 °C revealed irregular fine particles prone to agglomeration, indicative of the mechanical alloying process [9]. The SEM images displayed a particle size of 90 nm (Figure 2a), consistent with XRD data. Additionally, SEM coupled with EDX technology confirmed the presence of all elements within the compound, affirming its stoichiometry. Interestingly, the grain size observed in SEM images appeared larger than calculated by the Scherrer formula, suggesting multiple small crystallized grains within each observed grain (Figure 2b).
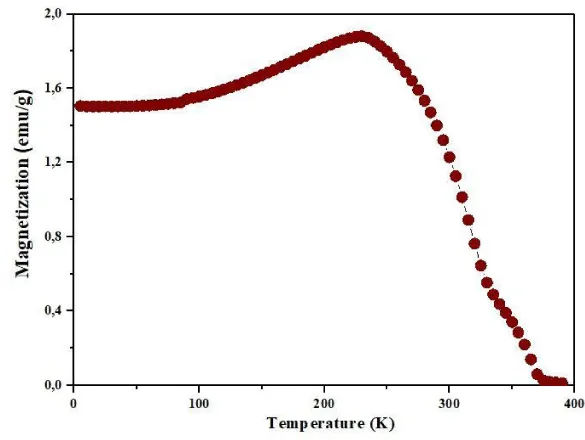
Magnetization, a key property in perovskite materials with the ABO3 crystal structure, was investigated in this study over a temperature range of 0 to 400 K. The results, depicted in Figure 3, revealed a notable increase in magnetization at approximately 375 K, indicating a transition from paramagnetic or antiferromagnetic to ferromagnetic states (Figure 3). In the ferromagnetic phase, magnetic moments align parallel, leading to net magnetization and the emergence of ferromagnetism [10-12]. Conversely, above 375 K, the material displays paramagnetic or antiferromagnetic behavior due to disordered or anti-aligned magnetic moments. The observed ferromagnetic transition has significant implications for potential applications, akin to transitions seen in other materials like barium titanate and strontium titanate at Curie temperatures of 393 K and 105 K, respectively.
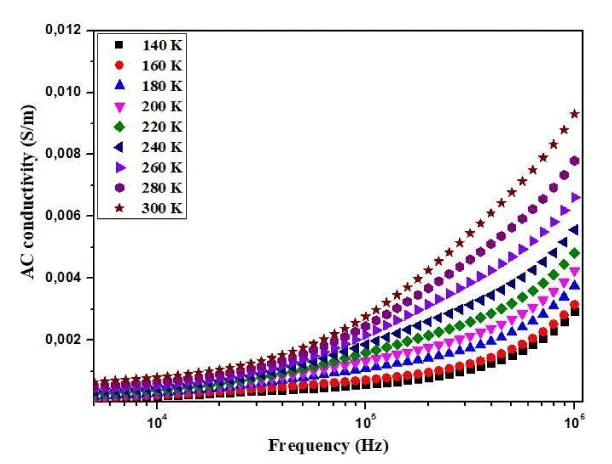
The examination of conductivity variation with alternating current (AC) frequency provides valuable insights into the charge transport mechanism and interactions among charge carriers. In Figure 4, the variation of this physical quantity concerning angular frequency at different temperatures is depicted. The conductivity spectra validate the existence of two distinct contributions: one at low frequencies, attributed to grain boundaries, and the other at high frequencies, associated with the grains. At low frequencies, conductivity maintains a constant and uniform profile, progressively increasing with temperature, indicating the activation of thermal conduction processes within the material [13].
Conclusion
In summary, the sol-gel synthesis of Sr0.6Ag0.4MnO3 perovskite resulted in a high-quality, monophasic material with a well-defined crystal structure (I4/mcm). Structural and morphological analyses confirmed purity and revealed a crystallite size of approximately 27 nm. Magnetic investigations demonstrated a distinctive ferro-paramagnetic transition at 375 K, showcasing its magnetic responsiveness. With its unique combination of magnetic and electrical properties, alongside its well-defined structure, Sr0.6Ag0.4MnO3 emerges as a promising candidate for various high-tech applications, including energy storage and electronics. This study significantly advances our understanding and exploration of perovskite materials tailored for diverse technological applications.
- Shaterian M. Synthesis, characterization and photocatalytic activity of LaMnO3 nanoparticles. Applied Surface Science. 2014; 318: 213-217. https://doi.org/10.1016/j.apsusc.2014.03.087.
- Tayari F. Sol-gel synthesized (Bi0.5Ba0.5Ag)0.5 (NiMn)0.5O3 perovskite ceramic: An exploration of its structural characteristics, dielectric properties, and electrical conductivity. Ceramics International. 2024; 50: 11207-11215. https://doi.org/10.1016/j.ceramint.2024.01.022.
- Benamara M, Iben Nassar K, Rivero-Antúnez P, Essid M, Soreto Teixeira S, Zhao S, Serrà A, Esquivias L. Study of Electrical and Dielectric Behaviors of Copper-Doped Zinc Oxide Ceramic Prepared by Spark Plasma Sintering for Electronic Device Applications. Nanomaterials (Basel). 2024 Feb 22;14(5):402. doi: 10.3390/nano14050402. PMID: 38470733; PMCID: PMC10935433.
- Iben NK. Structural, electrical properties of bismuth and niobium-doped LaNiO3 perovskite obtained by sol-gel route for future electronic device applications. Indian Journal of Physics. 2024: 1-9.
- Jamdar M, Monsef R, Ganduh SH, Dawi EA, Jasim LS, Salavati-Niasari M. Unraveling the potential of sonochemically achieved DyMnO3/Dy2O3 nanocomposites as highly efficient visible-light-driven photocatalysts in decolorization of organic contamination. Ecotoxicol Environ Saf. 2024 Jan 1;269:115801. doi: 10.1016/j.ecoenv.2023.115801. Epub 2023 Dec 7. PMID: 38064791.
- Tayari F. Structural, morphology, Raman spectroscopy, magnetic and electrical proprieties of BaNi0.5 Mn0.25Fe0.25O3 ceramic for electronic applications. Indian Journal of Physics. 2023; 97: 3545-3555.
- Benamara M. Light-enhanced electrical behavior of a Au/Al-doped ZnO/p- Si/Al heterostructure: insights from impedance and current-voltage analysis. RSC advances. 2023; 13: 28632-28641.
- Osinkin DA. An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique. Electrochimica acta. 2021; 372:137858. https://doi.org/10.1016/j.electacta.2021.137858.
- Nassar KI. Exploring bismuth-doped polycrystalline ceramic Ba0.75Bi0.25Ni0.7Mn0.3O3: synthesis, structure, and electrical properties for advanced electronic applications. RSC Advances. 2023; 13:24023-24030. https://doi.org/10.1039/d3ra05038f
- Heydariyan Z. EuMnO3/EuMn2O5/MWCNT nanocomposites: Insights into synthesis and application as potential materials for development of hydrogen storage capacity. Fuel. 2023; 351: 128885. https://doi.org/10.1016/j.fuel.2023.128885
- Khojasteh H. Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes. Diamond and Related Materials. 2017; 79: 133-144. https://doi.org/10.1016/j.diamond.2017.09.011
- Salavati-Niasari M, Mahnaz D, Fatemeh D. Pure cubic ZrO2 nanoparticles by thermolysis of a new precursor. Polyhedron. 2009; 28:14 3005-3009. https://doi.org/10.1016/j.poly.2009.06.032
- Tayari F, Iben Nassar K, Algessair S, Hjiri M, Benamara M. Investigating Fe-doped Ba0.67Ni0.33Mn1-xFexO3 (x = 0, 0.2) ceramics: insights into electrical and dielectric behaviors. RSC Adv. 2024 Apr 18;14(18):12561-12573. doi: 10.1039/d4ra01581a. PMID: 38638813; PMCID: PMC11024670.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
